Vol 9 No 1 2024-45
2024..09.01.45
Cytogenotoxic effect of trichothecene T2 toxin on Allium sativum root tip meristematic cells
Nasreen Jalal Hussein1 and Asia A. M. Saadullah2*
1Department of Biology/ College of Science/ University of Zakho, Zakho, Kurdistan Region/Iraq; nasreen.hussein@uoz.edu.krd.
2Department of Biology/ College of Science/ University of Duhok, Duhok, Kurdistan Region/ Iraq.
* Correspondence: asia.saadullah@uod.ac
Available from. http://dx.doi.org/10.21931/RB/2024.09.01.45
ABSTRACT
Trichothecene T2 is a mycotoxin from the Fusarium species. This research aims to test the effect of the Trichothecene T2 toxin on mitotic index% (M.I.%) and induction of mitotic aberrations by using the Allium sativum (garlic) test system. The toxin concentrations in ppm were 0.00, 0.30, 0.60, 0.90, and 1.20 for 12 hours. The garlic roots were then cut, and mitotic slides were prepared using squash and examined under a light microscope. The results revealed that the mycotoxin has a significant mitodepressive effect at all concentrations compared to the control, and the MI% reduction was proportional to increasing toxin concentration. The highest reduction in mitotic index was observed in the 1.2 ppm treatment.
Moreover, this mycotoxin induced and increased the rate of mitotic abnormalities% (MA%) with increasing the mycotoxin concentration. The observed mitotic abnormalities were star-shaped anaphase, sticky metaphase, C-mitosis, sticky anaphase, depolarization, micronuclei, laggard chromosomes, anaphase bridges, and chromosome loss. The least frequently observed abnormality was micronuclei compared to the most frequent aberration, laggard chromosomes. The total mitotic abnormalities significantly increased with increasing the toxin dose concentration. These results suggest that this mycotoxin can inhibit the mitotic activity of the meristematic cells; it is mutagenic and can disrupt the spindle fibers’ activity of the dividing cells at all concentrations, especially at higher doses in food. Therefore, the foods must be tested for fungi producing this mycotoxin.
Keywords: Mycotoxin; mitodepressive; root tip; spindle fibers; mutagenic
INTRODUCTION
Mycotoxins are natural chemicals fungus produces that infect cereals and other agricultural foods. They have a detrimental impact on animal and human health. They are also thermally stable and effortlessly transmitted to humans through animal products, sophisticated food preparation, and food chains1 toxic compounds are produced in foods and feeds by several strains of toxigenic fungi2, which ultimately cause health problems in humans and animals3. Trichothecenes (TCTs) are a family of chemically related mycotoxins produced most commonly by filamentous fungi such as Fusarium, Myrothecium, Stachybotrys, Trichoderma, and Trichothecium, Verticimonosporium, Cylindrocarpon, threatening the health of both animals and human4,5. The fungi that produce TCT are found all over the world. Their ability to grow under various environmental conditions, including nutrient, moisture, temperature, and oxygen levels in the growth medium, results in successful colonization6,7. T-2 toxin can be found in diverse regions worldwide and negatively affects human and animal health. It affects many organs and organ systems in various ways: neurotoxicity, immunotoxicity, hepatotoxicity, dermal toxicity, nephrotoxicity, reproductive system disruption, maternotoxic and embryo lethal8. T-2 toxin is thought to be a primary contributor in human alimentary toxic aleukia. TCT are non-volatile sesquiterpenoids with low molecular weight (usually 200-500 Da) produced by the terpenoid biosynthesis pathway9,10. They are somewhat soluble in water but very in polar organic solvents such as ethyl acetate, chloroform, ethanol, methanol, and propylene glycol1. TCTs share a three-ring molecule called 12,13-epoxytrichothec-9-ene (EPT) 11,12. The A-ring cyclohexene is fused to the B-ring tetrahydropyran, bridged by a two-carbon chain at C-2 and C-5, generating a cyclopentyl moiety (C-ring)13. TCT are classified into four kinds (A-D) based on the substitution pattern of EPT14. A hydroxyl (OH) group distinguishes type A TCT.
Define C-8 as having a group, an ester function, or no oxygen substitution15. Trichothecenes are quickly absorbed and suppress protein synthesis in growing tissues. Mycotoxins are natural chemicals fungi generate that infect grains and other agricultural goods. Trichothecenes mycotoxin produced by Fusarium and Trichoderma is becoming increasingly important in agriculture globally because of the potential health implications. The most dangerous type A trichothecene mycotoxin is T-2 toxin, an unavoidable environmental hazard. T-2 toxin has a considerable detrimental effect on reproduction. Plant systems like onion, garlic and beans are widely used to determine the cytotoxic and genotoxic effects of various chemicals like food additives, pesticides, and toxins16,17,18. Trichothecenes are important foodborne mycotoxins implicated in human health and have immune cytotoxic effects. Toxicity data on HT-2 toxin are minimal. To the best of the authors’ knowledge, no published data tests the cytogeneotoxic effects of this mycotoxin on A sativum root tip meristematic cells. Therefore, this study aims to test the Cytogenotoxic effect of T-2 mycotoxin on mitotic index and T-2 toxin-induced mitotic aberrations in A. sativum dividing cells at the root apical meristem.
MATERIALS AND METHODS
Garlic heads (Allium sativum, 2n=16) were purchased from a local store in Zakho, free from fertilizers.
Source of the mycotoxin T2 toxin
The mycotoxin used in the current study is the 8230 Veratox for T-2/HT-2 (NEOGEN company /USA), Cross Reactivity T-2 toxin 100%, HT-2 toxin B 100%. The product details are Veratox® for T-2/HT-2 is a competitive direct ELISA that provides a quantitative analysis of T-2 toxin and HT-2 toxin in commodities such as wheat, rye, barley, oats, and corn. The different concentrations were diluted by using distilled water.
The garlic was placed in tubes filled with distilled water for a few days until the roots emerged and reached a length of about 1 cm; then, they were removed from those tubes and placed on another set of tubes with different concentrations of the mycotoxin T2 for about 12 hours. Then, the roots were harvested, and microscopic slides for the root tip were prepared using the squash method, stained with Feulgen Giemsa stain, and visualized under light microscopy19.
The mitotic index calculation
The MI% calculation was done according to the following formula as published by Fiskesjö (1985).
M.I.%= DC/TC *100 (1)
Where, M.I.= Mitotic Index, DC=Dividing Cells, TC=Total counted Cells.
Mitotic abnormalities percentage
The mitotic abnormalities percentage was calculated by the following formula (Palsikowski et al., 2017)
MA%= ADC/TC*100 (2)
Where, MA=Mitotic Abnormalities, ADC=Abnormal dividing cells, TC=Total counted cells.
Statistical analysis
Most of the statistics were done using SPSS (Statistical Package for the Social Sciences) software version 14, by One way ANOVA, and differences between all the treatments were determined by Duncan’s multiple-range test 20. The data were calculated as mean ± standard error. Each experiment was repeated at least three times. The significance was measured at P<0.05. The bar graphs were generated using GraphPad Prism version 9.0.
RESULTS
Trichothecenes are a class of structurally related mycotoxins with different cytotoxicity degrees. Because of the potential health risks, trichothecene (TCT) mycotoxin is becoming increasingly important in agriculture around the globe. It is primarily metabolized and eliminated after consumption, producing more than 20 metabolites, the most important of which is the hydroxy trichothecenes-2 toxin. (A12). The effect of T2 toxin on mitotic index% is shown in Table 1 and Figure 1.
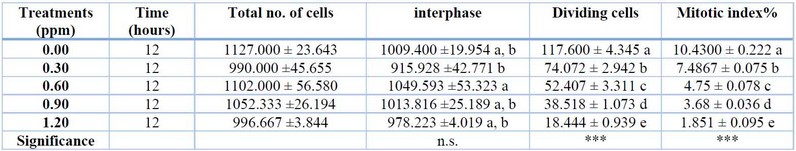
1 Note: n.s. means non-significant, *** means significant at p<0.001. DMRT produced different superscript letters.
Table 1: The effect of T2 toxin on the mitotic index% of A. sativum root tip cells.
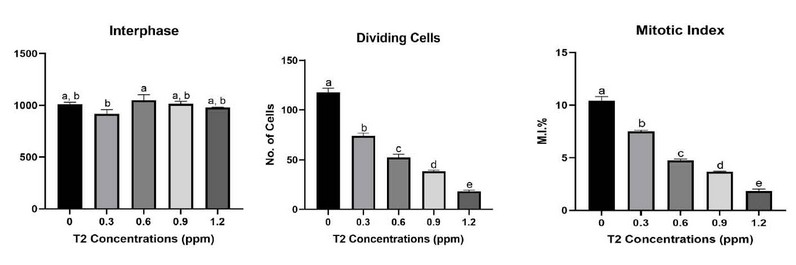
Figure 1: The effect of T2 toxin on no. of cells in interphase and dividing cells, and mitotic index% of A. sativum root tip cells.
The highest Concentration, 1.5 ppm which, caused it to decrease from 10.53 in the control to 0.7633 at 1.5 ppm. The mitodepressive effect was significant at P<0.001.
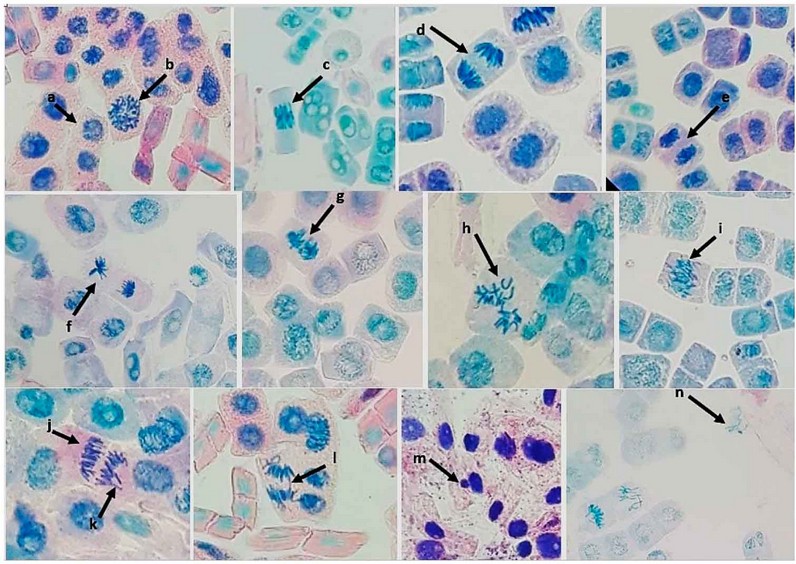
Figure 2: Normal mitosis and different abnormalities in A sativum root apical meristematic cells: a-normal interphase, b- normal prophase, c- normal metaphase, d- normal anaphase, e- normal telophase, f- Star-shaped anaphase, g- Sticky Metaphase, h- C-Mitosis, i- Sticky anaphase, j- Depolarization, k- Laggard Chromosomes, l- Anaphase Bridge, m- Micronucleus, n- Chromosome Loss.
The effect of the T2 toxin on chromosomal aberrations is shown in Table 2 and Figure 3. According to the Table, the aberrations increased proportionally with increasing mycotoxin concentration. Total mitotic abnormalities increased proportionally with increasing the Concentration of the mycotoxin. The observed mitotic abnormalities were star-shaped anaphase, sticky metaphase, C-mitosis, sticky anaphase, depolarization, micronuclei, laggard chromosomes, anaphase bridges, and chromosome loss. The least frequently observed abnormality was micronuclei at 0.00 in the control and increased to 0.1672 in the 1.2 ppm of T2. Compared to the most frequent aberration, laggard chromosomes were 0.0536% in the control and increased to 1.1341% in 1.2 ppm of the tested mycotoxin. The total CAI% was 0.1106 in the control group, which increased to about 5.2168% at the highest Concentration of the mycotoxin. Significant differences existed between treatments at p<0.001 for all mitotic aberrations.
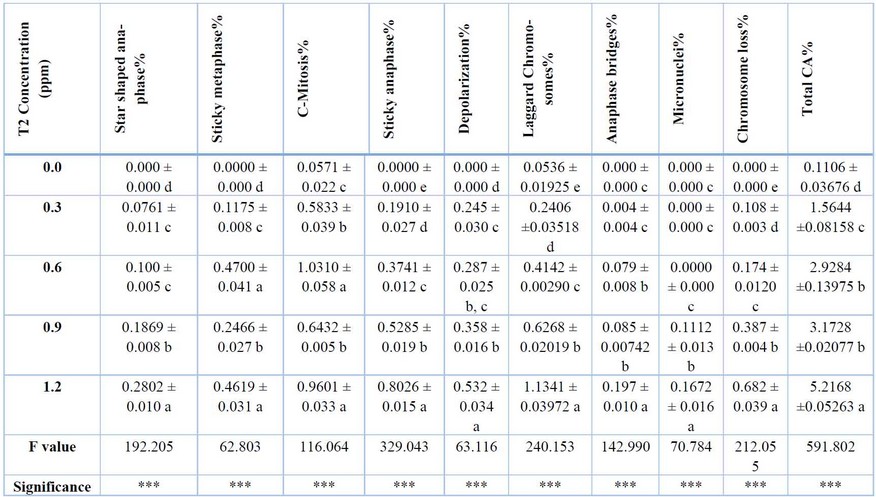
*** means significant at p<0.001. DMRT produces superscript letters
Table 2: the effect of T2 toxin on mitotic abnormalities% of A. sativum root tip meristematic cells after 12 hours.
The abnormalities caused by this mycotoxin in A. sativum root tip meristematic cells were star-shaped anaphase, sticky metaphase, C-mitosis, sticky anaphase, depolarization, micronuclei, and laggard Chromosomes. The most frequent abnormality was C-mitosis and sticky anaphase. According to Table 2, the total abnormalities increased with the increase in toxin concentration. The highest number was observed with the 1.5 ppm treatment. The different abnormalities and normal mitosis are shown in Figure 2. The mycotoxin T2 induces the distortion and inhibition of mitotic spindle formation, which causes the C-mitotic cells and stickiness in metaphase and anaphase. Moreover, the induction of chromosome loss and micronuclei.
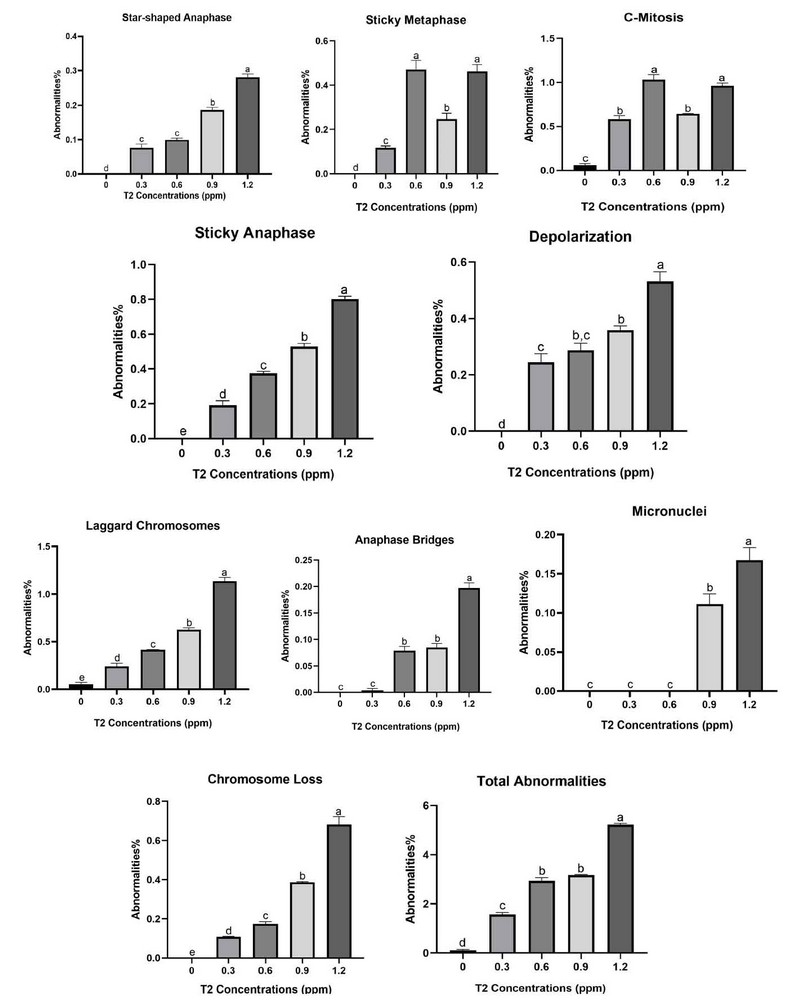
Figure 3: The effect of T2 toxin on mitotic abnormalities in A. sativum root tip meristematic cells.
About 1000 cells were counted for each slide, and each experiment was repeated thrice. According to Table 1, the different concentrations of mycotoxin caused a significant reduction in the mitotic index and the number of dividing cells at all treatments. However, the most significant effect on the mitotic index was observed at the highest Concentration of the mycotoxin treatment (1.2 ppm) which caused the mitotic index% to decrease from (10.4300 ± 0.222) in the control group to (1.851 ± 0.095) at 1.2 ppm treatment. The mitodepressive effect was significant at P<0.001. Moreover, there were substantial differences among the treatments as detected by DMRT.
The effect of the T2 toxin on chromosomal aberrations is shown in Table 2 and Figure 3. The abnormalities caused by this mycotoxin in A. sativum root tip meristematic cells were star-shaped anaphase, sticky metaphase, C-Mitosis, sticky anaphase, depolarization, micronuclei, and laggard chromosomes. The most frequent abnormalities were C-mitosis and sticky anaphase. According to Table 2, the total abnormalities were proportionally increasing with increasing toxin concentration. The highest number was observed with the 1.2 ppm treatment. The different abnormalities and normal mitosis, are shown in Figure 2.
The total MA% was (0.1106 ± 0.03676) in the control group, which increased to about (5.2168 ±0.05263) at the highest Concentration of the mycotoxin used. Significant differences existed between treatments at p<0.001 for all mitotic aberrations. The ability to induce different abnormalities in the A. sativum cells at the root apical meristem indicates its mutagenic activity.
DISCUSSION
According to Table 1 the different concentrations of mycotoxin caused a significant reduction in the mitotic index at all treatments. However, the greatest reduction in the mitotic index was observed at. other research tested the effect of Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division 21, and another research tested the toxic activity of citrinin, a fungal phytotoxin, and its mode of action in onion cells 22.
The abnormalities caused by this mycotoxin in A. sativum root tip meristematic cells were: star-shaped anaphase, sticky metaphase, C-Mitosis, sticky anaphase, depolarization, micronuclei, and laggard Chromosomes. The most frequent abnormality was C-mitosis and sticky anaphase. According to Table 2, the total abnormalities increased with the increase in toxin concentration. The highest number was observed with the 1.5 ppm treatment. The different abnormalities along with normal mitosis are shown in Figure, 2. The mycotoxin T2 induces the distortion and inhibition of mitotic spindle formation which causes the C-mitotic cells and stickiness in metaphase and anaphase. Moreover, the induction of chromosome loss and micronuclei.
The ability to induce different abnormalities in the A. sativum cells at the root apical meristem indicates its mutagenic activity. The induction of both Micronuclei and stickiness are the most obvious indicators of cytotoxicity. According to Table 2, the increase in the frequency of micronuclei was dose-dependent. These results are in agreement with the results obtained by 23. The total abnormality percentage increase was also dose-dependent. This increase agrees with previous results of testing various substances on onion root tips 24,25.
These results are in agreement with other research which tested the effect of Aflatoxin B1 on reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, and on inhibition of Allium cepa root cell division 26, and another research tested the toxic activity of citrinin, a fungal phytotoxin, and its mode of action in onion cells 27. The mycotoxin T2 induces the distortion and inhibition of mitotic spindle formation which causes the C-mitotic cells and stickiness in metaphase and anaphase. Moreover, the induction of chromosome loss and micronuclei. The least frequently observed abnormality was micronuclei at (0.000 ± 0.000) in the control and increased to (0.1672 ± 0.016) in the 1.2 ppm of T2. On the other hand, the most frequent aberration observed was laggard chromosomes were (0.0536 ± 0.01925) in the control and increased to (1.1341 ± 0.03972) in 1.2 ppm of the tested mycotoxin. A laggard chromosome is defined as a chromosome that did not overlap along the long axis of the spindle with any of the properly segregating chromosomes 28. The induction of both Micronuclei and stickiness are the most obvious indicators of cytotoxicity, Micronuclei are small membrane-bounded compartments with a DNA content encapsulated by a nuclear envelope and spatially separated from the primary nucleus (Krupina et al., 2021). Chromosome stickiness may result from chromatin fibers’ sticking to each other or breaking due to erroneous or inadequate condensation of these fibers, as a consequence of this, movement of mitotic spindle fibers together with inner-chromosome stickiness when the chromosome is drawn to the pole causes secondary anomalies (bridge and fragment occurrence) , Stickiness in chromosomes is an indication of the high toxicity of the chemical substance and usually this may kill the cells with the irreversible damages 25
CONCLUSIONS
The mycotoxin T2 is a mutagen that inhibits cell division and induces a wide range of abnormalities in A sativum root apical meristematic cells at lower doses and is very strong. Therefore, the food grains that contain this mycotoxin should be tested carefully to ensure that they are not contaminated with the T2 toxin-producing fungi to avoid its hazardous health risks.
REFERENCES
1. Polak-Śliwińska, M.; Paszczyk, B. 2021. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules. 26, 454.
2. Bryden, W. L. 2007. Mycotoxins in the food chain: Human health implications. Asia Pacific journal of clinical nutrition. 16:95–101.
3. Bennett, J. W., and Klich, M. 2003. Mycotoxins. Clinical Microbiology Reviews. 16:497–516.
4. Chen, P.; Xiang, B.; Shi, H.; Yu, P.; Song, Y.; Li, S. Recent advances on type A trichothecenes in food and feed: Analysis, prevalence, toxicity, and decontamination techniques. Food Control. 2020, 118, 107371.
5. Milićević, D.R.; Škrinjar, M.; Baltić, T. 2010. Real and Perceived Risks for Mycotoxin Contamination in Foods and Feeds: Challenges for Food Safety Control. Toxins. 2, 572.
6. McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. 2011. Trichothecenes: From simple to complex mycotoxins. Toxins. 3, 802–814.
7. Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. 2019. Trichothecenes in Cereal Grains—An Update. Toxins. 11, 634.
8. S K Kudury, I A Abed and B A Mahdi . The Effect of Local Bio Fertilizer and Their Enzymatic Activity on Growth of Maize Plant and some Biological Therites in the Soil. IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 012050. https://doi.org/10.1088/1755-1315/1252/1/012050.
9. Arunachalam, C., and Doohan, F.M. 2013. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicology Letters. 217, 149–158.
10. Bottalico, A., & Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Mycotoxins in Plant Disease: Under the aegis of COST Action 835 ‘Agriculturally Important Toxigenic Fungi 1998-2003′, EU project (QLK 1-CT-1998-01380), and ISPP’ Fusarium Committee’, 611-624.
11. Çavuşoğlu, D. 2022. Powerful toxic activity of citrinin, a fungal phytotoxin, and its mode of action in onion cells. Environmental Science and Pollution Research, 29(4): 6205-6218.
12. A A Al-Azzami , Th T Mohammed . Effect of Adding Dry Leaves of Lemongrass (Cymbopogon Citratus) To the Diet on Some Biochemical Tests of Blood in Broiler (Ross 308). IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12125. https://doi.org/10.1088/1755-1315/1252/1/012125.
13. Das, T., S. Hazra, S. Sengupta, P. Hazra, and D. Chattopadhyay. 2021. Genotoxic effect of saccharin on Allium cepa root tips. Biologia. 76: 3191-3199.
14. R S Obaid, S H S Al-Warshan and I A Abed . Efficiency of Some Clays and Organic Materials on the Reduction of Aflatoxin B1 Produced from Isolates of the Fungus Aspergillus flavus Contaminating Corn Grains. IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 012003. https://doi.org/10.1088/1755-1315/1252/1/012003.
15. Duncan, D. B. 1955. Multiple range and multiple F tests. Biometrics. 11: 1-42.
16. EFSA (European Food Safety Authority) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal. 2011:2481–2668. doi: 10.2903/j.efsa.2011.2481.
17. A A Al-Azzami , Th T Mohammed . The Effect of Adding Lemongrass Leaf Powder (Cymbopogon Citratus) to the Diet as a Natural Supplement on Some Productive Traits and Oxidation Indicators in Broiler (Ross 308). IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12123. https://doi.org/10.1088/1755-1315/1252/1/012123.
18. Foroud, N.A.; Shank, R.A.; Kiss, D.; Eudes, F.; Hazendonk, P. 2016. Solvent and Water Mediated Structural Variations in Deoxynivalenol and Their Potential Implications on the Disruption of Ribosomal Function. Frontiers in Microbiology. 7, 1239.
19. Garvey, G.S.; McCormick, S.P.; Rayment, I. Structural and Functional Characterization of the TRI101 Trichothecene 3-O-Acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum: Kinetic insights to combating fusarium head blight. Journal of Biological Chemistry, 283(3), 1660-1669.
20. Hussein, N. J. 2023. «Evaluation of Cytogenotoxic Effect of Potassium Acetate on Allium Cepa L. Root Tips». Emirates Journal of Food and Agriculture. 35(8):708-714. doi:10.9755/ejfa.2023.3129.
21. Ameen M. Shaman , Th. T. Mohammed. Effect Using Feed Additives Instead of Imported Premixes Affects the Physiology of Broiler Chickens. IOP Conf Ser Earth Environ Sci 2023, 1262 (7), 72080. https://doi.org/10.1088/1755-1315/1262/7/072080.
22. Jebur, S. F.; Abdulateef, S. M. Effect of The Critical Period and Gene Silencing on Blood Cellular Traits in Local Chicken and Level of Welfare. IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12141. https://doi.org/10.1088/1755-1315/1252/1/012141 .
23. Nielsen, C.; Casteel, M.; Didier, A.; Dietrich, R.; Märtlbauer, E. 2009. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Research. 25, 77–84.
24. Palsikowski, P. A., Roberto, M. M., Sommaggio, L. R., Souza, P. M., Morales, A. R., & Marin-Morales, M. A. (2018). Ecotoxicity evaluation of the biodegradable polymers PLA, PBAT and its blends using Allium cepa as test organism. Journal of Polymers and the Environment. 26: 938-945.
25. Pestka, J. J. 2007. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Animal Feed Science and technology. 137: 283–298.
26. Elkhateeb, S. Z.; Ebraheem, M. O.; Abdulateef, S. M.; Ahmed, I. A. Constraints Affecting the Welfare of Domestic Sheep Grazing in the Natural Pasture. IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12144. https://doi.org/10.1088/1755-1315/1252/1/012144.
27. Saadullah, A. A. M. (2022). Studies on teratogenic and maternal effects of Trichothecene (TCT) extracted from Fusarium and Trichoderma culture on pregnant Albino Mice. Revis Bionatura. 2023; 8 (2) 58.
28. Sharma, A. and A. Sharma. 1999. Chromosome Techniques. 3rd ed. Buterworths, London.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Hussein N. J. and Saadullah A. A. M. Cytogenotoxic effect of trichothecene T2 toxin on Allium sativum root tip meristematic cells. Revis Bionatura 2024; 9 (1) 45. http://dx.doi.org/10.21931/RB/2024.09.01.45
Additional information Correspondence should be addressed to asia.saadullah@uod.ac
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. First 13909355 Ecuador. Scopus coverage years: from 2016 to the present
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



















