Vol 8 No 4 2023 – 27
Comparison of ocular biometry and intraocular lens power using a novel biometer and a traditional biometer
Makarem Ali Hussain Alshammari 1* Siham sabah Abdullah Al Muhammad2
1 Al-Imam al-Sadiq Hospital.Iraq
2 Al-Nahrain Medicine Hopital. Iraq
*Corresponding authors: Email Makaremali3@gmail.com
Available from: http://dx.doi.org/10.21931/RB/2023.08.04.28
ABSTRACT
The implantation of an intraocular lens (IOL) is the gold standard in today’s cataract surgery. Calculating the power of the intraocular lens (IOL) has emerged as a central concern in cataract surgery over the last decade. The study aims to investigate the relationship between optical biometry and applanation ultrasound measurement of the eye’s axial length. This prospective cohort study was done on 60 eyes from sixty patients undergoing phacoemulsification with primary intraocular lens implantation and scheduled for cataract surgery in the Ophthalmology Department of al-Imam al-Sadeq Hospital. Thirty eyes of patients were measured by ultrasound measurement (by A-Scan, Group 1) and the other thirty eyes by optical biometry (by IOL Master, Group 2). In Group 1There were 14 eyes of 14 males (47%) and 16 eyes of 16 females (53%) with a mean age was 71.6 ±4.33years. In Group 2, there were 16 eyes of 16 males (53%) and 14 eyes of fourteen females (47%), and the mean age of the patients in this Group was 66.13±8.61 years. The mean IOL of the patient in Group I was (19.96±1.81). At the same time, the mean IOL potent ion of the patient in Group II was (22.96±1.66).
Keywords: Optical Biometry, Ultrasound Biometry, Intraocular lens (IOL), IOL Master
partial coherence interferometry (PCI)
INTRODUCTION
Calculating the intraocular lens (IOL) is an essential step in getting the precise aim that includes the refractive outcome and is a vital objective in recent cataract surgery1. Modern cataract surgery is a refined procedure that enables most patients to achieve high-quality postoperative vision. However, postoperative refractive outcomes remain a significant area of concern for surgeons due to advancements in surgical technique, new lOL technologies, improved biometric methods, and advanced methods of IOL power calculation 2.
Refractive error is no longer acceptable following cataract surgery due to improvements in technology for cataract surgery and the launch of distinctive intraocular lens implants. Consequently, precise biometric readings must be obtained for undistorted vision following surgery3.
Innovations like intraocular lens (IOL) power prediction algorithms, phacoemulsification, and ocular biometry have significantly improved the refractive result of cataract surgery during the last fifty years4. This result depends on precisely predicting the implanted IOL’s power, which relies primarily on preoperative biometry data, IOL power calculation methods, and manufacturer IOL power quality control. The most critical step for accurately calculating the IOL power is the preoperative measurement of the ocular axial length (AL)5.
A-scan ultrasonography, with a reported longitudinal resolution of approximately 200 μm and an accuracy of approximately 100–150 μm, is routinely employed to measure the ocular axile length AL. However, due to varying pressures the transducer exerts on the eye during applanation ultrasonography, which is often employed for ocular biometry, ultrasound biometry necessitates physical contact between the transducer and the eye 6. A postoperative refractive error of 0.28 diopters (D) is caused by an AL shortening of 0.1 mm in ultrasound biometry, and measurement errors in the AL have been shown to account for 54% of the expected refraction errors following IOL implantation.7 Recently, the IOL Master optical biometry device was created for commercial use based on the dual beam partial coherence interferometry (PCI) concept. The optical AL is measured using short-coherence infrared light (= 780 nm), and the geometric AL is then calculated using the group refractive index8. Additionally, it evaluates the corneal curvature, the anterior chamber depth, and the corneal diameter. Then, it determines the best IOL power using the biometry data it has collected and several IOL power calculation algorithms that are part of its computer software. The IOL master’s AL measures have exceptional precision, resolution, accuracy, and consistency 9.
In our study. We will look at the main factors that may affect the accuracy of refractive error prediction, how this residual error affects outcomes after surgery, and whether refractive outcomes improve with the new intraocular lens.
MATERIALS AND METHODS
All the patients underwent the following procedures: The information was gathered throughout the history-taking process: age, gender, domicile, and particular habits of the patients or their families. Relations, major complaint (painless progressive loss of eyesight), and examination of the complaint (onset, course, and duration). The Snellen visual acuity chart was completed for every patient before surgery. Preoperatively, patients received autorefraction, keratometry (K) and axial length measurement. Ultrasound B scans were performed on patients with dense media opacity and obscured fundus visibility. Non-cycloplegic autorefraction and fundus inspection were also performed. Assessment from the axial length of the eye included: Group I (Ultrasound A scan group, 30 eyes) had axial length measurements by A-scan ultrasound and K readings by manual keratometer. An optometrist does a scan-guided biometry. An accurate keratometric measurement is the first step in predicting IOL power. Next, the axial length was measured using the contact A-scan biometry. The patients were prepared by instilling one drop of tropicamide (mydriacil) 1%, then one drop of surface anesthesia in the form of benoxinate hydrochloride 0.4% was dropped into the patient’s eye. The data, including two keratometrics (K1, K2) and the average axial length plus a constant of IOL, were introduced in the calculating program of the optometrist. The intraocular lens power was based on the SRK/T formula, and all patients underwent uncomplicated cataract surgery by phacoemulsification within the bag IOL implantation through an interim precise corneal incision.
Statistical Methodology
Data analysis was carried out using the statistical package of Statistical Package for Social Sciences- version 25 (SPSS-25). Data were presented in simple percentages, mean, and standard deviation measures. The significance of the difference of different means (quantitative data) was tested using the student’s test for the difference between two unpaired tests. Scattering distribution curve used for correlation. Statistical significance was considered whenever the p-value was equal to or less than 0.05
RESULTS
Group 1 (A-scan ultrasound biometry group) Included 30 eyes subjected to biometry with A-Scan ultrasound biometry. There were 14 eyes of 14 males (47%) and 16 eyes of 16 females (53%). The mean age was 71.6 ± 4.33 years. Group 2 (Optical biometry (IOL Master group)) Included 30 eyes subjected to biometry with IOL Master Optical biometry. There were 16 eyes of 16 males (53%) and 14 eyes of fourteen females (47%), and the mean age of the patients in this Group was 66.13 ± 8.61 years. Also, a total of 60 eyes of 60 patients were enrolled in our study (eyes in 30 females (50%) and eyes in 30 males (50%). Group I: 14 eyes of 14 males (47%) and 16 eyes of 16 females (53%), and Group II: 16 eyes of 16 males (53%) and 14 eyes of fourteen females (47%), as shown in (table 1).
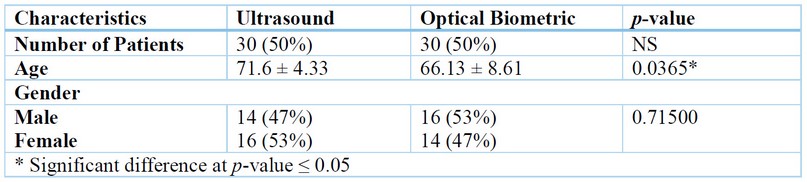
Table 1. Characteristics of Patients
The mean axial length of the patient in Group I was (22.55 ± 0.83 mm)
while II was (23.021 ± 0.71 mm) Also, the mean k1 reading of the patient in Group I was (44.1 ± 1.71) Diopter. While Group II was (44.68 ± 0.88) Diopter. Additionally, group I had a mean k2 reading of the patients as (44.81 ± 1.76) Diopter, and Group II had a mean k2 reading of the patients (45.45 ± 0.83) Diopter. Group I had a mean k reading of the patient of (44.45 ± 1.72) Diopter, whereas Group II had a mean of (45.07 ± 0.82) Diopter, as shown in (table 2).
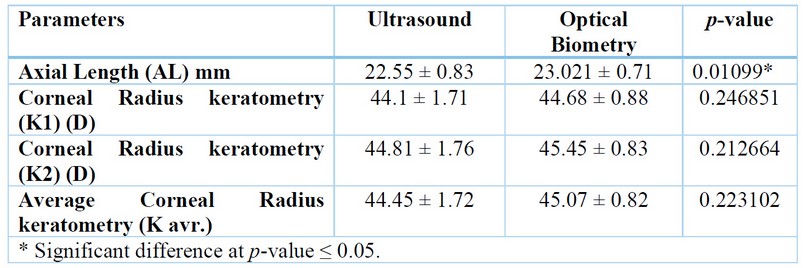
Table 2. The Axial Length and K reading distribution parameters between Ultrasound (US) and optical Biometry (OB)
Figure 1. Comparison between ultrasound (US) and optical biometry (OB) for Axial Length (AL).
The mean ACD of the patient in Group I was (2.93 ± 0.29) while, in Group, it was (3.21 ± 0.51) Also, the mean IOL of the patient in Group I was (19.96 ± 1.81) Whereas the mean IOL strength of the patient in Group II was (22.96 ± 1.66) Also, the mean lens thickness in Group I was (3.76 ± 0.78) While in Group II was (4.31 ± 0.41).
The spherical desired refraction of the patient in Group I was (-0.74 ± 0.04), and in Group II was (-0.91 ± 0.058) and achieved refraction of the patient in Group I
(-1.12 ± 0.02) Moreover, in Group II, it was (-1.05 ±0.01), as shown in (table 3).

Table 3. The Anterior Chamber Depth (ACD), Lens Thickness (LT), and the intraocular lens (IOL).
DISCUSION
Intraocular lens (IOL) measuring is crucial in modern cataract surgery to achieve the desired refractive outcome. The primary goal of cataract surgery is to restore clear vision by removing the cloudy lens and replacing it with a clear artificial lens. The correct power of the IOL is essential to achieve the desired refractive outcome and provide the patient with optimal visual acuity. Various technologies and formulas are available to accurately estimate the IOL power required for the desired refraction. Scan ultrasonography is the most commonly used method for measuring the ocular axial length (AL)14. However, modern technology and optical biometry have significantly improved our ability to accurately measure visual axial length (AL). The study included 60 eyes of 60 patients, with cataracts as the only ocular pathology and axial lengths less than 24.50 mm. The study group comprised 30 females (50%) and 30 males (50%). The patients were divided into two groups, with 30 patients implanted with an IOL calculated by the IOL master and 30 patients by ultrasound. The postoperative visual and refractive outcomes were evaluated in both groups. The patients implanted with the IOL calculated by the IOL master had a postoperative spherical refraction ranging from 0.01 to 1.05. In contrast, those implanted with an ultrasound had a postoperative spherical refraction ranging from 0.02 to 1.12. The results showed that the axial length measurements by optical biometry were 0.47 mm longer than those by A-scan ultrasonography (P<0.001). These findings are consistent with other studies that have reported similar differences. The most significant contributor to this disparity is the pressure the ultrasound probe exerts on the eye. Therefore, optical biometry is preferred over A-scan ultrasonography, as it provides more accurate measurements and reduces the risk of error. In conclusion, accurate IOL measurement is essential for achieving the desired refractive outcome in cataract surgery. Advances in technology and formulas have improved our ability to measure ocular axial length accurately. Optical biometry is the preferred method for measuring axial length over A-scan ultrasonography due to its accuracy and reduced risk of error. When measuring the length of the eye, there are two main methods: ultrasound and optical biometry. Both methods have their advantages and limitations. Ultrasound emits sound waves that penetrate the eye and bounce back to create an image.15 This image can then be used to measure the length of the eye. However, ultrasound has limitations in terms of accuracy and resolution. The accuracy of axial length with ultrasound is approximately 0.10–0.12 mm compared to 0.012 mm for optical biometry. This is because ultrasound is reflected mainly at the internal limiting membrane (ILM), while the light of the optical biometry is reflected at the retinal pigment epithelium (RPE). This results in a difference corresponding to the retinal thickness of the fovea, which is about 130 μm. On the other hand, optical biometry uses laser light to measure the length of the eye. Laser light has a very short wavelength compared to sound, which means it has better resolution.16 The light of the optical biometry is reflected at the RPE, which allows for a more accurate measurement of the length of the eye. However, the accuracy of both methods is limited by retinal thickness variation surrounding the fovea. A precise preoperative calculation is necessary to attain the most desirable results, and an exact IOL power formula must be employed. The refractive power of the human eye depends on the strength of the cornea keratometry (K) values, the axial length of the eye (AL), and the position of the lens. Therefore, the best refractive outcomes after surgery depend on accurately evaluating these factors. Patients may have a severe refractive error if these biometric measures and computations are wrong. This is why it is important for eye surgeons to carefully consider the measurement method and accurately evaluate the factors that contribute to the eye’s refractive power. Doing so can ensure that their patients have the best possible outcomes after surgery. Intraocular lens implantation is a common surgical procedure used to replace the natural eye lens with an artificial one. The success of this surgery depends on accurate measurements of the eye’s dimensions, including axial length and keratometry readings. These measurements help determine the appropriate size and power of the intraocular lens to be implanted, which affects the patient’s postoperative vision. Unfortunately, these measurements can lead to significant refractive errors after surgery, negatively impacting the patient’s visual acuity and quality of life. According to recent studies, errors in assessing the influential lens position (ELP) are responsible for most of these postoperative refractive errors. Incorrect ELP assessment accounts for 38% of these errors, while keratometry error accounts for only 8%. This highlights the importance of accurate ELP assessment in achieving optimal refractive outcomes after intraocular lens implantation.17One way to improve the accuracy of these measurements is through optical biometry, which measures axial length using light instead of sound waves. Studies have shown that optical biometry is more accurate than traditional A-scan ultrasound measurements, with the IOL Master measuring axial length 0.47 mm longer than A-scan measurements. This difference was statistically significant, indicating that optical biometry may be a more reliable method for measuring axial length. However, not all studies have shown a significant difference between optical biometry and A-scan ultrasound measurements. In a recent study, GAD et al. found that the difference in axial length measurements between these two methods was only 0.34 mm, which was not statistically significant. This suggests that the choice of measurement method may depend on individual patient factors and the surgeon’s preference. Similarly, studies have shown varying results when comparing keratometry readings obtained through different methods. In our study, we found that the mean average K reading (Kav) measured by a keratometer used with A-scan was 44.45±1.72 D, while the mean Kav measured by the IOL Master was 45.07±0.82 D. This difference was statistically significant, indicating that the choice of keratometry method may also affect postoperative refractive outcomes. In conclusion, accurate assessment of axial length and keratometry readings is crucial for achieving optimal refractive outcomes after intraocular lens implantation. While optical biometry may be a more accurate method for measuring axial length, the choice of measurement method may depend on individual patient factors and surgeon preference. Similarly, the keratometry method may also affect postoperative refractive outcomes. Further research is needed to determine the most reliable methods for these measurements and to improve the success rate of intraocular lens implantation surgery. Corneal power measurement is a crucial step in determining the appropriate intraocular lens (IOL) power for cataract surgery. In recent years, there has been a shift towards using optical biometry, such as the IOL Master, to measure corneal power rather than traditional ultrasound techniques. This is due to the greater accuracy and precision of the IOL Master, which measures corneal power using a six-point measure on a smaller diameter circle than traditional ultrasound techniques. The bell-shaped cornea is a unique feature that must be considered when measuring corneal power. As the cornea flattens towards the eye’s periphery, measurements taken with an auto-keratometer using a ring 3 millimeters in diameter centered on the cornea may not provide the most accurate results. The IOL Master’s more central measurement approach better accounts for the cornea’s shape and produces more clinically relevant results. A recent study found that the IOL power measured by the IOL Master was significantly more substantial than ultrasound techniques. This difference was statistically significant and highlights the importance of using optical biometry for measuring corneal power. However, other studies have found discrepancies between the IOL Master and other measurement techniques, such as the A-scan, which provided more extraordinary IOL powers. Despite these discrepancies, the SRK-T formula remains widely used and reliable for calculating IOL power. More accurate and precise methods for measuring corneal power will likely emerge as technology advances. In the meantime, using the most reliable and clinically relevant techniques available to ensure successful outcomes for cataract surgery patients is essential. The anterior chamber depth (ACD) measurement has long been a crucial step in ocular biometry. Traditionally, this measurement was taken using actual measurements of the ACD, which were based on assumptions that short eyes would have shallower ACDs and long eyes would have deeper ACDs. However, this method was not entirely accurate and often resulted in errors in estimating intraocular lens (IOL) power, leading to poor postoperative refractive outcomes. Fortunately, advancements in technology have significantly contributed to simplifying the ocular biometry procedure. One such advancement is the IOLMaster, a non-contact approach that does not require topical anesthetic. This provides comfort to the patient and reduces the risk of corneal abrasions and spreading infections. Optical biometry, using Swept-source OCT, like the IOLMaster, provides a measurement based on an image that enables the operator to observe a whole longitudinal eye segment. The operator can monitor the fovea’s picture and be warned if the patient is not appropriately fixating. This alerts the operator to any irregular eye geometries, such as lens tilt, allowing for more precise estimations of the IOL power and ultimately resulting in improved postoperative refractive conditions. In addition to measuring the ACD, the IOLMaster is now taking the place of ultrasonography in measuring the axial length (AXL). It offers rapid data capture and the ability to measure the AL along six distinct axes, making it a more efficient and accurate biometry method. Despite technological advancements, however, ultrasonic biometry cannot be ruled out entirely, as some eyes, anywhere from 8% to 10% of cases, continue to require it. Therefore, a combination of various biometry methods may be necessary to ensure accurate and reliable measurements for successful cataract surgery outcomes. Various factors can often hinder the collection of accurate optical AXL data. One such factor is corneal scarring, which can cause opacity in the cornea, making it difficult to obtain clear measurements. Similarly, opaque cataracts can impede the collection of optical AXL data, as they can obstruct the visual axis and prevent the instrument from accurately measuring the length of the eye. Another factor that can lead to erroneous AXL measurements is poor fixation, particularly in cases where the patient suffers from age-related macular degeneration. In such cases, the patient may be unable to maintain optimum fixation, leading to inaccurate readings. 18 Moreover, positioning disabled patients on the IOL Master machine can be a challenging task that requires special consideration. Such patients may have physical limitations that make it difficult to assume the required position for the instrument to take accurate measurements. As such, special measures must be taken to ensure that these patients are comfortably and safely positioned on the machine without compromising the accuracy of the AXL measurements. In conclusion, collecting optical AXL data can be a complex process that requires careful consideration of various factors. By understanding the potential challenges during the process, medical professionals can take the necessary precautions to ensure that accurate measurements are obtained and that patients receive the best possible care.
CONCLUSIONS
The IOL Master is a cutting-edge technology that has revolutionized the field of ophthalmology. With its simple and easy-to-use interface, the IOL Master has become a favorite among eye care professionals for its ability to measure the eye’s axial length precisely, which is crucial for accurate intraocular lens (IOL) power calculations. Unlike traditional methods of measuring the eye, the IOL Master does not require the patient to make eye contact, which is especially helpful for those with difficulty with eye movements or limited mobility. Additionally, the IOL Master is entirely safe and eliminates the risk of transmitting diseases through contact, making it an ideal tool for use in any healthcare setting. One of the most significant advantages of the IOL Master is its ability to provide more accurate IOL power calculations. By taking precise measurements of the eye’s axial length, the IOL Master helps ensure that the IOL selected for a patient has the correct power and size, resulting in improved postoperative refractive conditions. This means patients can enjoy better visual acuity and enhanced quality of life after cataract surgery. Overall, the IOL Master is a powerful tool that offers numerous benefits for patients and eye care professionals. Its ease of use, safety, and accuracy make it an invaluable asset in diagnosing and treating eye conditions. As technology continues to evolve, the IOL Master will remain at the forefront of ophthalmic innovation for years.
REFERENCES
1. Committee MSA. Optical Biometry Using Partial Coherence Interferometry Prior to Cataract Surgery. Canberra Dep Heal Aging. 2005;
2. Abdelghany AA, Alio JL. Surgical options for correction of refractive error following cataract surgery. Eye Vis. 2014;1(1):1–7.
3. Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol Scand. 2007;85(5):472–85.
4. Findl O, Drexler W, Menapace R, Heinzl H, Hitzenberger CK, Fercher AF. Improved prediction of intraocular lens power using partial coherence interferometry. J Cataract Refract Surg. 2001;27(6):861–7.
5. Steinert RF. Cataract surgery: Expert Consult-Online and Print. Elsevier Health Sciences; 2009.
6. Byrne SF, Green RL. Ultrasound of the eye and orbit. Mosby Incorporated; 2002.
7. Santodomingo-Rubido J, Mallen EAH, Gilmartin B, Wolffsohn JS. A new non-contact optical device for ocular biometry. Br J Ophthalmol. 2002;86(4):458–62.
8. Gaballa SH, Allam RSHM, Abouhussein NB, Raafat KA. IOL master and A-scan biometry in axial length and intraocular lens power measurements. Delta J Ophthalmol. 2017;18(1):13.
9. Eleftheriadis H. IOLMaster biometry: refractive results of 100 consecutive cases. Br J Ophthalmol. 2003;87(8):960–3.
10. Elsaadani AEI, Badawi NM, Elterawy AZA. Optical biometry versus ultrasound biometry. Egypt J Hosp Med. 2020;81(5):2088–92.
11. Alpins NA, Walsh G, Optom В. accurate biometry and intraocular lens power calculations. Refractive Surgery Nightmares-Conquering Refractive Surgery Catastrophes. Slack Inc; NJ; 2008. p. 581–5.
12. Drexler W, Findl O, Menapace R, Rainer G, Vass C, Hitzenberger CK, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126(4):524–34.
13. Iyamu E, Osuobeni E. Age, gender, corneal diameter, corneal curvature and central corneal thickness in Nigerians with normal intraocular pressure. J Optom. 2012;5(2):87–97.
14. Gad MN, Mourad MS, Rehan RA, Mustafa MM. Intraocular lens Master optical biometry vs conventional ultrasonic biometry in intraocular lens power calculations in highly myopic vs emmetropic eyes. J Egypt Ophthalmol Soc. 2020;113(2):39.
15. Moon SW, Lim SH, Lee HY. Accuracy of biometry for intraocular lens implantation using the new partial coherence interferometer, AL-scan. Korean J Ophthalmol. 2014;28(6):444–50.
16. Meena DS, Sukhadia H, Goyal S, Kumar V. Compare the Accuracy of IOL Power Calculations Using Ultrasound Biometry and Partial Coherence Laser Interferometry based Optical Biometry. Indian J Forensic Med Toxicol. 2021;15(2):120–6.
17. Shajari M, Kolb CM, Petermann K, Böhm M, Herzog M, de’Lorenzo N, et al. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J Cataract Refract Surg. 2018;44(8):942–8.
18. Ferrer-Blasco T, Domínguez-Vicent A, Esteve-Taboada JJ, Aloy MA, Adsuara JE, Montés-Micó R. Evaluation of the repeatability of a swept-source ocular biometer for measuring ocular biometric parameters. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:343–9.
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation. Hussain Alshammari, Siham M A, Al Muhammad S A. Comparison of ocular biometry and intraocular lens power using a novel biometer and a traditional biometer. Revis Bionatura 2023;8 (4) 28. http://dx.doi.org/10.21931/RB/2023.08.04.28
Additional information Correspondence should be addressed to Makaremali3@gmail.com



















