Vol 8 No 3 2023 – 62

Procedure for obtaining flour from Canavalia ensiformis (L) seeds
Rodríguez – Quiñones, Pavel1 , Aurora Terylene Pérez Martínez2
, Aurora Terylene Pérez Martínez2 , Dayami Fontes Marrero3
, Dayami Fontes Marrero3 , Liannis Pérez Gómez4
, Liannis Pérez Gómez4 , Marcos Edel Martínez Montero5*
, Marcos Edel Martínez Montero5* , Denisse Margoth Zambrano Muñoz6
, Denisse Margoth Zambrano Muñoz6 , Natalys Solis7
, Natalys Solis7 and Cristóbal Ismael Bolaños Velez8
and Cristóbal Ismael Bolaños Velez8 
 , Aurora Terylene Pérez Martínez2
, Aurora Terylene Pérez Martínez2 , Dayami Fontes Marrero3
, Dayami Fontes Marrero3 , Liannis Pérez Gómez4
, Liannis Pérez Gómez4 , Marcos Edel Martínez Montero5*
, Marcos Edel Martínez Montero5* , Denisse Margoth Zambrano Muñoz6
, Denisse Margoth Zambrano Muñoz6 , Natalys Solis7
, Natalys Solis7 and Cristóbal Ismael Bolaños Velez8
and Cristóbal Ismael Bolaños Velez8 
1 Departamento Procesos Agroindustriales. Facultad de Ciencias Agropecuarias. Universidad de Ciego de Ávila Máximo Gómez Báez.
2 Centro de Bioplantas. Universidad de Ciego de Ávila Máximo Gómez Báez.
3 Departamento Producción Agrícola. Facultad de Ciencias Agropecuarias. Universidad de Ciego de Ávila Máximo Gómez Báez.
4 Centro de Bioplantas. Universidad de Ciego de Ávila Máximo Gómez Báez.
5* Departamento Procesos Agroindustriales. Facultad de Ciencias Agropecuarias. Universidad de Ciego de Ávila Máximo Gómez Báez.
6 Facultad de Ciencias de la Industria y Producción. Universidad Tecnica Estatal de Quevedo.
7 Facultad de Ciencias Agropecuarias, Universidad Técnica de Ambato;
8Gestor Ambiental. Provincia Santa Elena.
Correspondence: cubaplantas@gmail.com; Tel.:5358818116
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.62
ABSTRACT
Legume seeds are rich in protein, making them useful in various biotechnological processes, such as protein hydrolysates, with different uses based on biological activity. These bioproducts can be used as agricultural biostimulants due to their ability to improve nutrient absorption and assimilation processes, biotic and abiotic stress tolerance, and agronomic indicators of crops. Among tropical legumes, Canavalia ensiformis (L.) is a promising crop because of its adaptability to various soil and climate conditions, high germination rate, and agricultural yield. This study aimed to establish a technological procedure for producing C. ensiformis seed flour to use as a protein source for agricultural biostimulant production. The seeds underwent an 84-h imbibition process in running water, and the water absorption index and percentage of seeds that experienced plasmolysis were determined, with indicators evaluated every 12 h. The seed coat was removed, and the cotyledons were dried in an oven at 60°C for 24 h, establishing the drying curve and productive yield. The resulting flour from the 72-h imbibition process was analyzed for particle size, techno-functional and chemical properties, which were compared to the flour of whole seeds. The study showed that the procedure improved the techno-functional and chemical properties of the flour and its effectiveness in obtaining flour easily and cost-effectively.
Keywords: absorption, imbibition process, flour, seeds.
INTRODUCTION
The amino acid-based biofertilizers constitute an important source of essential elements for plant development, facilitating their assimilation into plant tissues. The increasingly frequent application of biostimulants in the agricultural sector improves food safety and nutritional quality.1 The use of raw materials derived mainly from plants is an alternative for their use as multifunctional materials.2
On the other hand, legumes are an essential source of proteins, from which value-added products can be obtained, such as protein hydrolysates or peptides with different biological activities.3 Among the families of tropical legumes, the genus Canavalia has 12 species with agricultural potential, among which Canavalia ensiformis (L.) can be found. This legume has a high capacity for adaptation to climatic and edaphic conditions.4 C. ensiformis is a tropical legume with a protein content higher than 26%, belonging to the Fabaceae family.5
Hydrating legumes with distilled water reduces the concentrations of inhibitors in beans, such as trypsin, tannins, phytic acid, and saponins.6 Reductions of these antinutritional factors have been reported by processing methods, such as gamma radiation, dehulling, imbibition, sprouting, boiling, malting, and fermentation.7 The water absorption and retention capacity are techno-functional properties of dry legumes. These determine the appropriate imbibition times of grains and are directly related to the protein content and protein-water interaction.8 Although the benefits of grain imbibition in water are scientifically recognized, this practice is not used massively.9
Given the background above, seeking a procedure according to the existing technological capabilities for obtaining C. ensiformis seed flour is necessary, considering its contribution to proteins, which can be used in biotechnological processes to produce agricultural biostimulants. Therefore, the objective can be to establish a procedure for creating C. ensiformis seed flour using the imbibition method for biotechnological processes.
MATERIAL AND METHODS
The research was conducted in 2022 at the Plant Physiology Laboratory facilities of the University of Ciego de Ávila Máximo Gómez Báez, Cuba. Seeds of the Canavalia ensiformis (L.) species were used, which were cultivated at the Juan Tomas Roig Experimental Station belonging to the Bioplants Center of the University of Ciego de Ávila Máximo Gómez Báez and collected in December 2021.
Developed technological procedure
The C. ensiformis seeds were subjected to different evaluations during the successive technological stages of the process to determine the viability of the proposed procedure. This procedure aims to obtain flour for later use as a protein source in the production of agricultural biostimulants. The evaluated process consists of four technological stages (Figure 1).

Figure 1. Technological procedure for the production of C. ensiformis seed flour.
To obtain flour from C. ensiformis seeds, the imbibition method was used, which involved immersing the seeds in running water. During the seed imbibition process, the water absorption index concerning time was evaluated at intervals of 0, 12, 24, 36, 48, 60, 72, and 84 h. The mass-to-volume ratio was 1:20, with 1 g of fresh seed mass in 20 mL of running water placed in 500 mL beakers (Figure 1). The new seed mass was determined using a technical balance (Sartorius model BSA 2201, China) with a margin of error of 0.1 g. Five replicates were made, each with 12 seeds.

Figure 2. Imbibition of Canavalia ensiformis seeds in running water.
Once the imbibition process was completed, the seeds were dried with an absorbent material (filter paper), and the dry mass of the seeds was determined. The water absorption index was calculated using the formula:
Water Absorption Index = fresh mass ∂t / fresh mass (t0)
Subsequently, the seed coat was manually removed, and the percentage of seeds showing plasmolysis was determined. The drying process was carried out in an electric oven with digital temperature control (Brand Boxun, China) at 60°C for 24 h, and the drying curve of the C. ensiformis cotyledons was defined. The moisture loss during the drying process was evaluated every 2 h. The values taken to create the drying curve were referred to as the moisture loss index (Δ Moisture) using the equation:
Δ Moisture = {1 – [massa (fresh) – massa (dry) / massa (dry)]}
The dry seeds were ground in a manual disc mill, and the industrial yield, qualitative parameters, techno-functional properties, and chemical properties of the C. ensiformis flour were evaluated concerning fresh mass.
Qualitative characterization, techno-functional properties, and chemistry of C. ensiformis seed flour
To determine the qualitative characteristics of the C. ensiformis flour (particle size), 100 g were placed in a sieve shaker (Ro-tap-SDILTEST-model CL-313-8, Italy) with a set of standard sieves and kept under agitation for 15 min. Finally, the fractions of flour retained on each sieve were weighed, and the retention percentage was calculated. The final mean size value was obtained from five replicates. The resulting flour was analyzed for crude protein content using the Kjeldahl method10, soluble proteins determined by the Bradford method11, and total phenols by reaction with the Folin-Ciocalteu reagent.12 Also, the techno-functional properties, such as water-holding and foam-forming capacity, were determined as described by Bravo.13 Water holding capacity values correspond to the grams of water held per gram of flour. The foam forming capacity was expressed (mL/mL) as the volume of the foamed layer/total volume. The evaluated parameters were compared with flour from seeds without water imbibition treatment (control treatment).
Statistical processing of the data
For statistical analysis of the data, the SPSS software was used. Normal distribution was determined using the Kolmogorov-Smirnov test, homogeneity of variance was tested using Levene’s trial, and the significance of means was determined using the Student’s t-test, one-way ANOVA, and Tukey’s test with a significance level of P ≤ 0.05. Percentage data were transformed using the mathematical equation y’ = 2arcsin(√y/100) for statistical analysis.
RESULTS
Table 1 shows the relationship between imbibition time and the absorption index of C. ensiformis seeds. It can be seen that imbibition time directly affects the absorption index, as there is an increase in source fresh mass over time.
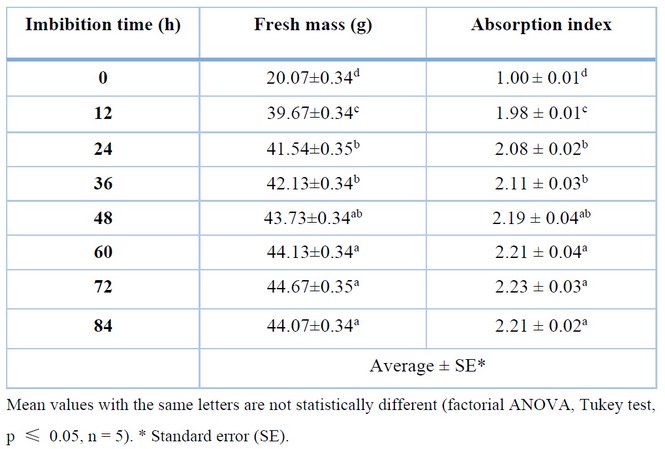
Table 1. Effect of imbibition time on the absorption index of Canavalia ensiformis seeds.
In the control treatment (0 h), there was no gain in seed mass as there was no water absorption, resulting in an absorption index of 1.00 at 12 h, indicating a significant increase in seed mass due to water absorption. Between 12 and 24 h, there was a substantial increase in the absorption index, resulting in statistically significant differences between their means. After 24 h, there were no significant variations in the absorption index concerning 36 and 48 h, as well as between 48, 60, 72, and 84 h, except for the significant differences between 36 and 60 h. Although there were no statistically significant differences between 72 and 84 h, there was a slight decrease in the absorption index at 84 h, which may have been caused by the presence of seeds that underwent plasmolysis, the solubility of some substances present in the seeds, and the onset of natural fermentation.
After the imbibition process and as a preliminary step to drying, the seminal envelope of C. ensiformis seeds was manually removed. The effect of imbibition time on the percentage of plasmolysis of the seeds can be seen in Table 2. Plasmolysis facilitates the seed coat removal more easily and quickly than seeds that do not undergo this process. An indicator of plasmolysis is that seeds under this condition float in the liquid medium, and degradation of the seed coat is observed, indicating the onset of a fermentation process.
Table 2 shows that no seeds presented plasmolysis in the control treatment and at 12, 24, and 36 h. In the 48-h treatment, 8% of the seeds gave plasmolysis (Figure 3), indicating a statistically significant difference in treatments evaluated with shorter imbibition time. The treatment assessed at 60 h showed 21.3% of seeds with plasmolysis, with substantial differences compared to 48 h. From 72 h, an increase in the percentage of seeds with plasmolysis was observed, reaching a value of 78.7%, indicating a significant difference concerning 60 h. At 84 h, the rate of sources with plasmolysis was 81.3%, and no statistically significant difference was found concerning 72 h.
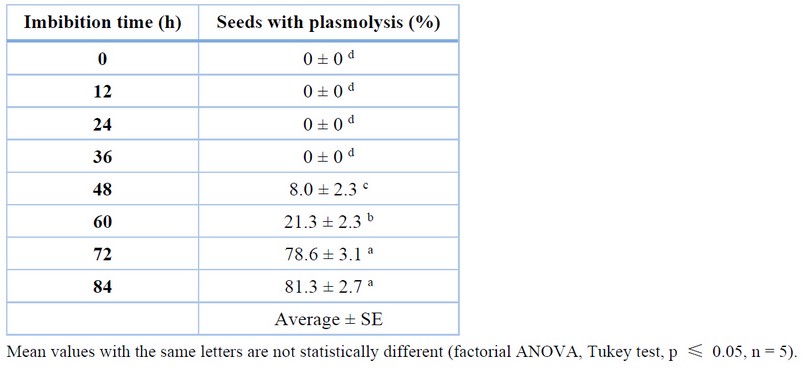
Table 2. Percentage of C. ensiformis seeds presenting plasmolysis during the soaking process.

Figure 3. Plasmolysis in C. ensiformis seeds, degradation of seed coat and initiation of a fermentation process
Given that the percentage of plasmolysis reaches 78.6% at 72 h and there is no significant difference compared to 84 h, and the absorption rate of the seeds between 48 and 84 h shows no significant differences among their means, it can be inferred that the optimal imbibition time is 72 h. Therefore, evaluating the flour’s physical, chemical, and techno-functional properties subjected to the imbibition process should be based on seeds imbibed for 72 h.
Figure 4 shows the dynamics of water loss from the dehulled cotyledons of C. ensiformis seeds over time. The graph indicates a more significant trend in the drying process during the first 6 h, as their means differ significantly. From 14 h, the mass stability begins, with a mean value that does not differ significantly from the standards at 16 and 18 h, but there is a significant difference between 14 h and 20 h. From 18 h, there are no significant differences in the mean value compared to the means at 20, 22, and 24 h. Therefore, it can be concluded that the optimal drying time is 18 hours.

Figure 4. Dynamics of moisture loss index of C. ensiformis seed cotyledons without seed coat in the time interaction. Mean values with the same letters are not statistically different (factorial ANOVA, Tukey test, p ≤ 0.05, n = 5).
After undergoing absorption, manual removal of the seed coat, and drying processes, the cotyledons were ground using a manual disk mill, and the particle size of the resulting flour was evaluated. In the control treatment, the seeds from which the seed coat could not be removed could not be ground using the manual disk mill. Table 3 presents the results of the sieving analysis of the flours. The first sieve (#4) showed no retention of flour, as the sieve opening was more significant than the particle diameter. Sieves #8 and #16 had retention percentages of 3.72 and 9.85 %, respectively. The retention percentages in sieve #30, #60, and #80 were 20.52, 13.73, and 28.58 %, respectively, while the final collector retained 23.60 % of the sample. These values indicate that 86.43 % of the model has a particle size smaller than 1180 μm, demonstrating uniformity in particle size.

Table 3. Granulometry of C. ensiformis flour.
After the drying and grinding process, the productivity yield was 73.7 ± 0.1% concerning the initial mass of the seeds (fresh mass), resulting in losses of 26.2%. These losses can be attributed to the removal of the seed coat and the soluble microelements that passed into the liquid medium due to the plasmolysis of the seeds. The high incidence of seeds with plasmolysis may have led to the onset of spontaneous fermentation due to the presence of microorganisms in the aqueous medium. The resulting product has a fine texture, bone-white color, and a characteristic odor.
The obtained flour was analyzed for its soluble and total proteins and phenolic concentration (Table 4) and techno-functional properties related to water-holding capacity and foam formation capacity (Table 5) to evaluate its nutritional and functional characteristics.

Table 4. Chemical properties of C. ensiformis seed flour.

Table 5. Techno-functional properties of C. esiformis flour.
The study analyzed flour’s soluble protein content and phenol concentration obtained from both non-imbibed and imbibed seeds. The results showed that the soluble protein content was significantly higher in the imbibed seed flour (197.3 mg/g) compared to the non-imbibed seed flour (19.6 mg/g). On the other hand, the phenol concentration was significantly lower in the imbibed seed flour (60.2 µg/g) compared to the non-imbibed seed flour (99.0 µg/g).
Regarding techno-functional properties, the water-holding capacity remained the same for both non-imbibed and imbibed seed flours, indicating that imbibition and seed coat removal did not affect the water-holding ability of the flour. However, the foam formation capacity decreased significantly after the imbibition process, which could be attributed to the changes in the protein structure caused by the imbibition process.
Overall, the results suggest that imbibing seeds can significantly increase the soluble protein content of the resulting flour without affecting its water-holding ability. However, it can also lead to a decrease in the foam formation capacity of the flour.
DISCUSIONS
According to Megat-Rusydi et al.14, germination and imbibition of seeds result in the activation of hydrolytic enzymes, which initiate the breakdown of macromolecules such as proteins and carbohydrates, directly modifying the techno-functional properties of flours. During imbibition, metabolic processes in seeds start with ATP production and respiratory activity, which begin a few minutes after the process initiates. However, in the process described in this research, seeds cannot start respiratory activity after absorbing water in the imbibition process due to being in an aqueous environment. This interrupts the germination process initiated in the 1 h of imbibition and causes plasmolysis in the seeds, facilitating the seed coat removal. The beginning of seed plasmolysis coincides with the stability of water absorption at around 48 h. According to Vira Putri et al.15, the multiplication of microorganisms is represented by foam on the water’s surface during the imbibition phase of C. ensiformis seeds. This effect can be observed in this research. This is because it has been discovered that imbibed legumes contain lactic acid bacteria and yeast.16
According to Rios et al.17, flour with high particle size uniformity absorbs water homogeneously and promotes even cooking, which is beneficial for biotechnological processes. These characteristics facilitate greater homogeneity in protein extraction, making it possible to optimize operations such as enzymatic protein hydrolysis.
The flour of C. ensiformis has a different particle size than the flour evaluated by Dussán-Sarria et al.18, who studied this characteristic in chontaduro and quinoa flours. Compared to chontaduro flour, C. ensiformis flour has smaller particle diameters, is more homogeneous, and does not form lumps. Compared with quinoa flour, it has a larger particle size and less homogeneity in its composition. However, the percentage of particles smaller than 250 μm in both flours was similar, with 52.18% in C. ensiformis flour and 50% in quinoa flour.
El-Suhaibani et al.19 suggested that imbibition can increase the availability of amino acids in certain foods by breaking down antinutrients such as phytic acid and tannins. This is supported by Vira Putri et al.15, who observed a significant increase in certain hydrophobic amino acids in seeds. Depending on the processing method used, these biochemical changes can impact the techno-functional properties of resulting flours. The highest levels of foaming capacity and essential amino acids in C. ensiformis flour were observed after 72 h of germination.15 This increase can be attributed to more excellent protein solubility, given the increased levels of free amino acids.20 While the cited authors suggest that the germination process increases the foaming capacity of flours, the process described in this research shows that this techno-functional property decreases during the 72-h imbibition process.
According to studies by Vira Putri et al.15, C. ensiformis seeds after 24 h of soaking have a protein content of 30%, indicating a small reduction in concentration compared to soaking for 12 h, while hulled seeds contained 32%. According to a study by Kanetro et al.21, the protein content of jack beans increased after 36 and 48 h of imbibition. The authors’ findings are consistent with the data from this research, which showed protein percentages of 27.3% for whole seeds and 28.8% for seeds imbibed for 72 h and subsequently dehulled. Other authors reported a significant decrease in the protein content of C. ensiformis after imbibition, which could be attributed to the release of ammonia from amino acid deamination during leaching.22
Regarding ash content, it was demonstrated that its percentage decreased as imbibition time was prolonged. The process of boiling, imbibing in water, and dehulling could cause a 41% loss of ash that can remain in the grain husk or leach during processing.23 According to Ramli et al.24, the nutrient and antinutrient compositions of C. ensiformis subjected to an imbibition pretreatment with 1% NaHCO3 reduced antinutrients such as HCN, phytic acid content, saponins, tannins, and oxalates. These results are consistent with the research conducted, where the imbibition process of seeds in running water decreased the concentration of total phenols. The technological process described improves the techno-functional properties of C. ensiformis seed flour by reducing its foaming capacity. This makes the flour obtained in a subsequent enzymatic hydrolysis process have less foam formation and, therefore, less protein loss due to this cause.
CONCLUSIONS
Based on the experimentation and subsequent analysis and evaluation of the data obtained, the viability of the process can be concluded. The resulting C. ensiformis seed flour has a fine texture, bone-white color, and a characteristic odor. The evaluated soaking-drying procedure is an effective and economical way to obtain flour, with a productive yield of 73.7%. The soaking process improves the techno-functional properties of the flour by maintaining its water absorption capacity and decreasing foam capacity. The described process also enhances the total protein content and keeps the amount of soluble proteins while reducing the concentration of phenols. Therefore, the evaluated procedure is suitable for using this flour in biotechnological processes, such as producing enzymatic protein hydrolysates.
Acknowledgments: The authors would like to express their gratitude to the staff of the Faculty of Agricultural Science and Centre of Bioplantas of the University of Ciego de Ávila Máximo Gómez Báez for their invaluable support and assistance throughout this research project. Their expertise and resources were instrumental in ensuring the success of this study, and the authors sincerely appreciate their contributions.
Conflicts of interest: The authors declare no conflict of interest.
REFERENCES
1. Ovalle-Torres BS, Barrallaza-Torres O, Peña-Peña E. Producción y caracterización de bioestimulantes para la producción agrícola a partir de residuos locales. Rev Electr ANFEI Dig. 2019;6(11).
2. Gasca-Tuz C, Chel-Guerrero L, Betancur-Ancona D. Capacidad antibacteriana de fracciones peptídicas de frijol lima (Phaseolus lunatus L.) obtenidas por hidrólisis enzimática. JONNPR. 2017;2(1):8-16. doi:10.19230/jonnpr.1150
3. Kina-Ysa M, Flores-Fernández CN. Obtención de hidrolizados proteicos de leguminosas usando una pro-teasa recombinante de Pseudomonas aeruginosa M211. Rev Chil Nutr. 2020;47(3):381-389. doi:10.4067/S0717-75182020000300381
4. Cáseres O, González E, Delgado R. Canavalia ensiformis: leguminosa forrajera promisoria para la agricultura tropical. Pastos y Forrajes. 1995;18(2):107-119.
5. Solomon SG, Okomoda VT, Oguche O. Nutritional value of raw Canavalia ensiformis and its utilization as partial replacement for soybean meal in the diet of Clarias gariepinus (Burchell, 1822) fingerlings. Food Sci Nutr. 2017;6(1):207-213.
6. García Pacheco Y, Cabrera Mercado D, Ballestas Santos JA, Campo Arrieta MJ. Efecto de diferentes tratamientos térmicos sobre las propiedades tecno-funcionales de la harina de fríjol blanco (Phaseolus lunatus L.) y la determinación de su potencial uso agroalimentario. INGE CUC. 2019;2(15):132-142. doi:10.17981/ingecuc.15.2.2019.13
7. Osman AMA, Hassan AB, Osman GAM, Mohammed N, Rushdi MAH, Diah EE, Babiker EE. Effects of gamma irradiation and/or cooking on nutritional quality of faba bean (Vicia faba L.) cultivars seeds. J Food Sci Tech. 2012;51:1554-1560.
8. Sánchez M, Ruiz J, Dávila G, Jímenes C. Propiedades Tecnofuncionales y Biológicas de Harina, Aislado y Fracciones Proteicas Mayoritarias de Semillas de Inga Paterno. J Food. 2017;15(3):400-408. doi:10.1080/19476337.2017.1286522
9. Serna-Cock L, Pabón-Rodríguez OV, Quintana-Moreno JD. Efectos de la Fuerza Iónica y el Tiempo de Remojo de Legumbres Secas sobre sus Propiedades Tecnofuncionales. Inf Tecnol. 2019;30(2):201-210. doi:10.4067/S0718-07642019000200201
10. AOAC International. Official Methods of Analysis of AOAC International, 20th Edition. Techtreet. Available online: https://www.techtreet.com/standards/official-methods-of-analysis-of-aoac-international-20th-edition-2016?product_id=1937367 (accessed on 12 june 2023).
11. Bradford M. A rapid and sensitive method for quantitation of submicrogram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. http://hoffman.cm.utexas.edu/courses/bradford_assay.pdf
12. Gurr SI, McPherson MI, Bowles DJ. Lignin and associated phenolic acids in cell walls. Mol Plant Pathol Pract Approach. 1992;3:62-69.
13. Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56(11):317-333. doi:10.1111/j.1753-4887.1998.tb01670.x
14. Megat-Rusydi MR, Noraliza CW, Azrina A, Zulkhari A. Nutritional changes in germinated legumes and rice varieties. Int Food Res J. 2011;18(2).
15. Vira Putri Y, Djali M, Andoyo R, Nizam L, Mohd M, Rifqi M. Processes. 2023;11(1161). doi:10.3390/pr11041161
16. Yan Y, Wolkers-Rooijackers J, Nout MJR, Han B. Microbial diversity and dynamics of microbial communities during back-slop soaking of soybeans as determined by PCR-DGGE and molecular cloning. World J Microbiol Biotechnol. 2013;29:1969-1974.
17. Rios MJB, Damasceno-Silva KJ, Moreira-Araújo RSD, Figueiredo EAT, Rocha MDM, Hashimoto JM. Chemical, granulometric and technological characteristics of whole flours from commercial cultivars of cowpea. Revista Caatinga. 2018;31(1):217-224. doi:10.1590/1983-21252018v31n125rc
18. Dussán-Sarria S, Hurtado-Hurtado DL, Camacho-Tamayo JH. Granulometría, Propiedades Funcionales y Propiedades de Color de las Harinas de Quinua y Chontaduro. Inf Tecnol. 2019;30(5):1-9. doi:10.4067/S0718-07642019000500003
19. EL-Suhaibani M, Ahmed MA, Osman MA. Study of germination, soaking and cooking effects on the nutritional quality of goat pea (Securigera securidaca L.). J King Saud Univ – Sci. 2022;32:2029-2033.
20. Kanetro B. Amino acid profile of soybean (Glycine max) sprout protein for determining insulin stimulation amino acids. Int Food Res J. 2018;25:2497-2502.
21. Kanetro B, Riyanto M, Pujimulyani D, Huda N. Improvement of functional properties of Jack Bean (Canavalia ensiformis) flour by germination and its relation to amino acids profile. Curr Res Nutr Food Sci. 2021;9:812-822.
22. Monika, Savitri, Kumar V, Kumari A, Angmo K, Bhalla TC. Isolation and characterization of lactic acid bacteria from traditional pickles of Himachal Pradesh, India. J Food Sci Technol. 2017;54:1945-1952.
23. Omafuvbe BO, Falade OS, Osuntogun BA, Adewusi SRA. Chemical and biochemical changes in African locust bean (Parkia biglobosa) and melon (Citrullus vulgaris) seeds during fermentation to condiments. Pakistan J Nutr. 2004;3:140-145.
24. Ramli NAM, Chen YH, Mohd Zin Z, Abdullah MAA, Rusli ND, Zainol MK. Chemical characterization and source identification of groundwater in a tropical island using multivariate statistical techniques. IOP Conf Ser Earth Environ Sci. 2021;756:012033. doi:10.1088/1755-1315/756/1/012033.
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Rodríguez – Quiñones, P, Pérez Martínez A T, Fontes Marrero D, Pérez Gómez L, Martínez Montero M E, Zambrano Muñoz D M, Solis N and Bolaños Velez C I. Procedure for obtaining flour from Canavalia ensiformis (L) seeds. Revis Bionatura 2023;8 (3) 62. http://dx.doi.org/10.21931/RB/2023.08.03.62



















