Vol 7 No 3 2022- 47

Antifungal Activity and Qualitative Phytochemical Analysis of Green alga Ulothrix sp.
Department of Biology, College of Education For Pure Science, University of Mosul/Mosul- Iraq
*Corresponding Author: zainulabdeen.hamzah@uomosul.edu.iq, mobile :+964 7701610400
Available from: http://dx.doi.org/10.21931/RB/2022.07.03.47
ABSTRACT
The antifungal activity of the ethanolic hot extract of the green filamentous species Chlorophyta was evaluated in vitro at various doses (25, 50, and 100 mg/ml) and shown to be effective. Antifungal activity was performed by evaluating the percentage inhibition growth method against some fungus were obtained from the postgraduate laboratories in the Department of Biology – Faculty of Science / University of Mustansiriya (Aspergillus niger, Fusarium oxysporum, Penicillium sp. and Rhizoctonia solani ). As a result of the research, it was discovered that the hot ethanol extract of Ulothrix sp had the most significant effect (91.8 %) on Rhizoctonia solani growth inhibition at a concentration of 100 mg/ml and the minor effect (22.4 %) at a concentration of 25 mg/ml against Aspergillus niger growth inhibition. It was discovered by primary chemical analysis of active substances that alkaloids, Terpenes, Saponines, phenols, Flavones, Resins, Steroids, and tannins were present in hot ethanolic alga extract. Finally, the GC-mass analysis performed on Ulothrix sp extracts revealed a large number of antibacterial activity-producing substances. Because the current research shows that algae have antifungal activity, it has the potential to be developed as a new source of active chemicals for human and plant consumption in a variety of applications shortly.
Keywords: Antifungal activity, Ulothrix, Active compounds,
INTRODUCTION
The green alga Ulothrix sp., which belongs to the family Ulothricaceae, includes about 30 species and is generally found in fresh and marine water.Thallus has the advantage which are long, filamentous, un-branched, multicellular, having a single line of cells (uniseriate) and attached to the substratum by a holdfast1,2. Antibiotics are used in an indiscriminate and illogical manner, and this is one of the causes that contribute to the formation of antibiotic-resistant strains of bacteria. The search for new and natural sources that have the efficacy of antimicrobial resistance. Algae consider being a potentially active source of antimicrobial and antioxidant compounds 3,4. Because algae is an alternative source of many of diseases of cancer and other infectious diseases because of their chemical and biological diversity 5,6.
MATERIALS AND METHODS
Sample Collection and Preparation
Ulothrix specimens were collected. The sample was collected from aspiring of water in Tarjella village with Al-Hamdaniya District within Nineveh governorate, This station is located on longitude 43°28’33″E and latitude 36°19’49″N during spring 2021 figure (1). For the transfer to the laboratory, samples were placed in plastic bags. The samples are cleaned from dirt and substance by washing carefully with tap water and then drying for three days in the sun 7.

Figure 1. Collection region of the sample. At 36°19’49″N and 43°28’33″E 7Km
Soxhelet proses
According to 8,9 convert the dried powder of macroalgae ethyl alcohol to get hot alcoholic extract by Soxhelet extraction for six hours. When the extraction process is complete, evaporation increases concentration for an hour at 50˚C, which is then stored in sanitized test tubes until future usage.
Antifungal susceptibility
The antifungal activity of the macro-algae was investigated first by mixing different concentrations of the crude extracts of the macro-algae with Potato Dextrose Agar (PDA) medium 10, 11 to obtain different concentrations (25, 50, and 100) mg/ml and inoculating each plate with a block disk of fungal mycelia (1cm in diameter) and allowing it to grow at 2± 28°C for seven days in the dark. It was discovered that there was radial growth. For each treatment, three duplicate plates were utilized in total. According to the formula, the percentage of fungal inhibition was determined by testing.
% Inhibition = (Cd-Td) x 100/Cd
Where,
Cd = The diameter of the control colony (in millimeters).
Td = Test plate colony diameter measured in millimeters (mm).
The percentage of inhibition was computed, as well as an analysis of variance for each treatment option.
Qualitative estimation of active compounds
The standard protocols determined of presence and absence of active compounds from Ulothrix specimens12.
Gas Chromatography-mass Spectrometry (GC/MS):
GC/MS It is the method of choice for separating smaller and more volatile compounds in a sample, but it is also the most expensive because of its complexity. The characteristics of an Agilent Technologies (SHIMADZU / Japan) high-temperature column (Inert cap 1MS; 30 m x 0.25 mm id x 0.25 mm film thickness) were investigated. Using a high-temperature column, we were able to remove the necessity for the derivatization of each sample. 280°C was selected as the temperature for the injector and detector, while 100°C was set as the temperature for the starting column in the experiment. It was decided to run the column in split (1:10) mode with a 5 μl sample volume injected into it. A ramp rate of 12.5°C/min was used to increase the oven temperature to 225°C after one minute (hold time four minutes), and then a ramp rate of 7.5°C/min was used to raise the oven temperature to 300°C after five minutes (hold time five minutes). GC-Mass Solution and Postrun software, both of which can be downloaded from the Agilent website, were used to record and analyze the mass spectra and keep the helium carrier gas flowing at a constant rate of 17.5 ml/min. It was determined which chemicals were involved by comparing their mass to those found in the NIST library and legitimate standards 11.
RESULTS
The green algae Ulothrix sp. isolate from Tarjella village with Al-Hamdaniya District within Nineveh governorate, cells consist of unbranched, cylindrical, uniseriate filaments figure (2). These findings agreed with 1,2.
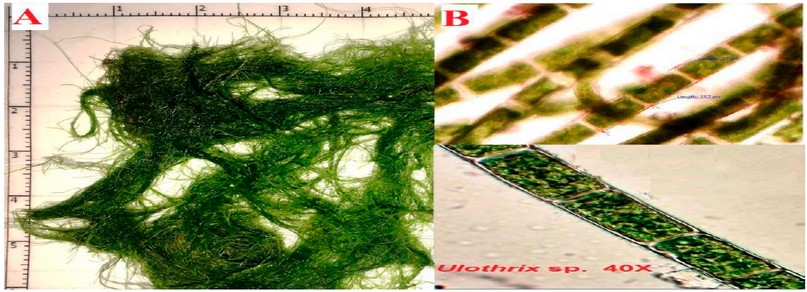
Figure 2. Filaments of Ulothrix specimens. ( A ) Length of filament in nature ( B ) unbranching under the microscope at 40X.
The antifungal activity of the ethanolic hot extract was evaluated based on the validity of experimental data of percent inhibition obtained against fungi, which were used to determine the extract’s efficacy (Ulothrix sp). Table (1) and Figure (3) shows the inhibitory of ethanolic hot extract (Ulothrix sp ) against fungi. It shows more high antifungal activity due to fungi and concentration of algal extract. The percentage of inhibition ranged at 100mg/ml concentration between (85.4-91.8) %. The highest value (91.8)% in Rhizoctonia solani. While the lower value (85.4)% at Penicillium sp. The percentage of inhibition ranged at 50 mg/ml concentration between (60.3-80.2) %. The highest value (80.2) % was in Rhizoctonia solani. But, the lower value (60.3)% at Penicillium sp. Finally, The percentage of inhibition ranged at 25 mg/ml concentration between (22.4-66.2)%. The highest value (66.2)% was in Rhizoctonia solani. On the other hand, the lower value (22.4) % was for Aspergillus niger. The findings revealed a flexible relationship between algal extract concentrations and the percentage inhibition of fungi; nevertheless, when the concentration of algal extract is high, the percentage of fungal inhibition is also high.

Table 1. Percentage inhibition growth of different fungal pathogen by using ethanolic hot extract of Uolthrix sp. at different concentration.

Figure 3. Growth inhibition of Rhizoctonia solani on PDAM plates by using ethanolic hot extract (Ulothrix sp ) at 100 mg/ml concentration A: Control. B: Ethanolic hot extract (Ulothrix sp. ) at 100 mg/ml.
Evaluation of Phyto-active compounds
The findings indicated that the hot alcohol extract of Ulothrix sp. included a significant amount of saponins, tanins, alkaloids, phenols, and flavonoids. Additional metabolites, such as glycosides and terpenoids, were not detected in the extracted sample; the findings are summarized in Table (2).

Table 2. Active components of Ulothrix sp ethanolic hot extract
Evaluations of Gas Chromatography-mass Spectrometry:
The GC-MS study of the Ulothrix sp. extract revealed various compounds, some of which were ignored because they did not include eight compounds, and of these only three significant components accounted for 85.6 % of the total mass (Fig. 4). Due to its scarcity, the remaining 14.4 % composition could not be determined. Table (3) lists the principal discovered chemicals ethanolic hot extract of Ulothrix sp. Alkane hydrocarbons Nonadecane (16.2%) and Pentadecane (39.5%) were identified in Ulothrix sp hot ethanolic crud.

Figure 4. Ulothrix sp. extract chromatogram of GC-Mass spectrophotometery that combination of at least 8 chemicals

Table 3. Major active compounds of green alga Ulothrix sp extract by using GC-Mass spectrophotometer.
DISCUSSION
In different studies, the alga extract has been demonstrated to exhibit antibacterial activity both in vivo and in vitro; in the past three decades, there has been a significant surge in the discovery of algae-derived metabolites having biological activity13. In addition to their other features, these compounds exhibit various biological actions, including antibacterial, antiviral, and antifungal, insecticidal, and antiproliferative effects 14,15. Interestingly, it was shown that hot ethanolic alga extract exhibited antifungal efficacy at all doses, which is consistent with previous findings 16; it was discovered that macro-algae extracts were efficient against the majority of the tested fungus, including Botryotrichum piluliferum, Fusarium oxysporium, and Alternaria brassicicola, when extracted in methanol or ethyl acetate. There have been several research on the antibacterial activity of algal extracts that have been mentioned 5,1. Results from these studies are difficult to compare because the antimicrobial activity of algae extracts can be influenced by many factors, including the algal species used in extraction, testing methodologies and the type of solvent used in extraction, the amount of time or period that samples were stored, and the thallus regions that were used 17,18. Mammals and plants benefit from algae-derived bioactive chemicals, which have been demonstrated to protect them against biotic and abiotic stressors by enhancing their defense mechanisms. Mammals and plants benefit from the use of biologically active chemicals (antimicrobial activity, hunting of free radicals and host defense activity etc.); it has been discovered that the extract contains an alkane hydrocarbon known as Cn-H2n+2. The conclusions of this study are consistent with past studies on this issue 19. It has previously been observed that a variety of marine algae include straight-chain paraffin (n-alkanes), divided-chain paraffin (alkyl-alkanes), and unsaturated hydrocarbons (alkenes) 19,20. Octadecane, Tetradecane, and hexadecane were discovered as standard major volatile components in all algal extracts, and these results were verified when compared to other examined hydrocarbons 21. A vast number of researches on the methodologies for production and the configuration of algal extracts have lately been published. Extract composition is highly dependent on both the source material (geographic location of macro-algae and algal species collected) and the extraction process used. From the algal biomass to the molten phase, polyphenols, polysaccharides, proteins, polyunsaturated fatty acids, minerals, pigments, plant growth hormones, and other physiologically active substances are transported. Humans, animals, and plants can all benefit from their well-predicted benefits, which include protection against environmental and internal stresses (such as antibacterial activity, free radical scavenging, and host defense), and they can be found in a variety of pharmaceuticals, feed additives, and dietary supplements 22,23.
CONCLUSION
Because they include a variety of active compounds that affect fungus development directly, algal Ulothrix extracts have shown dramatic suppression when used against several soil-borne pathogens, generally. Antifungal chemicals might be generated from this.
.
Funding: self-funding.
Acknowledgments I would like to convey my gratitude to the University of Mosul’s president for providing the necessary laboratory and other resources for this study to be completed.
Conflicts of Interest: Nil
REFERENCES
1. Sundara Rajan, S .Introduction to Algae (Anmol Publications PVD 2001. Ltd.,New Delhi,.
2. Sheath RG, Wehr JD. Introduction to the freshwater algae. In Freshwater Algae of North America 2015 Jan 1 (pp. 1-11). Academic Press. http://dx.doi.org/10.1016/B978-0-12-385876-4.00001-3
3. Ibtissam C, Hassane R, Jose M, Francisco DS, Antonio GV, Hassan B, Mohamed K. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. African Journal of Biotechnology. 2009;8(7).1258-1262
4. Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Science and Technology. 2008;41(6):1067-1072.
5. Munir N, Rafique M, Altaf I, Sharif N, Naz S. Antioxidant and antimicrobial activities of extracts from selected algal species. Bangladesh Journal of Botany. 2018 Mar 1;47(1):53-61.
6. Lavanya R, Veerappan N. Antibacterial potential of six seaweeds collected from Gulf of Mannar of southeast coast of India. Advances in Biological Research. 2011;5(1):38-44.
7. Karm LI, Dwaish AS. Investigation of Some Ecological Factors and Isolation Techniques for Some Local Algae in Iraq. Annals of the Romanian Society for Cell Biology. 2021:1059-1068.
9. Stein EM, Colepicolo P, Afonso FA, Fujii MT. Screening for antifungal activities of extracts of the Brazilian seaweed genus Laurencia (Ceramiales, Rhodophyta). Revista Brasileira de Farmacognosia. 2011;21(2):290-295.
10 Abdulwahid KE, Dwaish AS, Dakhil OA. Green synthesis and characterization of zinc oxide nanoparticles from Cladophora glomerata and its antifungal activity against some fungal isolates. Plant Arch. 2019;19(2):3527-3532.
11. Dwaish MS. Anti-dermatophytes activity of some algal extracts isolated From Baghdad City-Iraq. Research Journal of Pharmacy and Technology. 2018;11(12):5449-5454.
12. Frey D, Oldfield RJ, Bridger RC. Color atlas of pathogenic fungi. Year Book Medical Publishers; 1979.Ltd.168pp
13. De Quiros AR, Lage-Yusty MA, López-Hernández J. Determination of phenolic compounds in macroalgae for human consumption. Food Chemistry. 2010;121(2):634-638.
14. Yousif DY, Dwish AS, Shafiq SA. Antifungal activity of algal Spirogyra sp. against fungal Fusarium oxysporum. W. J Pharm. Res. 2014;4(1):1620-1628.
15. Kausalya M, Rao GN. Antimicrobial activity of marine algae. J. Algal Biomass Utln. 2015;6(1):78-87.
16. Majula E, Rao GM. In vitro study of antimicrobial activity in marine algae Caulerpa Taxifolia and Caulerpa Racemosa. International Journal of Applied Biology and Pharmaceutical Technology. 2014;5(2):57-62.
17. Veeragurunathan V, Geetha T. Screening for antimicrobial activity of marine algae from Gulf of Mannar, Tamil Nadu. Seaweed Res. Utilin. 2009;31(1&2):151-155.
18. Maréchal JP, Culioli G, Hellio C, Thomas-Guyon H, Callow ME, Clare AS, Ortalo-Magné A. Seasonal variation in antifouling activity of crude extracts of the brown alga Bifurcaria bifurcata (Cystoseiraceae) against cyprids of Balanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplanktis. Journal of Experimental Marine Biology and Ecology. 2004;313(1):47-62.
19. Oumaskour KH, Boujaber NA, Etahiri SA, Assobhei OM. Anti-inflammatory and antimicrobial activities of twenty-three marine red algae from the coast of Sidi Bouzid (El Jadida-Morocco). Int. J. Pharm. Pharm. Sci. 2013;5:145-149.
20. Youngblood WW, Blumer M, Guillard RL, Fiore F. Saturated and unsaturated hydrocarbons in marine benthic algae. Marine Biology. 1971;8(3):190-201.
21. Gelpi E, Schneider H, Mann J, Oro J. Hydrocarbons of geochemical significance in microscopic algae. Phytochemistry. 1970;9(3):603-612.
22. Tellez MR, Schrader KK, Kobaisy M. Volatile components of the cyanobacterium Oscillatoria perornata (Skuja). Journal of Agricultural and Food Chemistry. 2001 Dec 17;49(12):5989-5992.
23. Dwaish AS, Yousif DY, Lefta SN. Use of Spirogyra sp. extract against multi drug resistant bacterial pathogens. International Journal of Advanced Research. 2016;4(7):575-579.
Received: 13 March 2022 / Accepted: 25 July 2022 / Published:15 August 2022
Citation: Zainulabdeen H. A. AL-Khafaji. Antifungal Activity and Qualitative Phytochemical Analysis of Green alga Ulothrix sp. Revis Bionatura 2022;7(3) 47. http://dx.doi.org/10.21931/RB/2022.07.03.47




















