Vol 4 No 2 2019 – 12
NEWS AND VIEWS /NOTICIAS Y OPINIONES
The structure of Neurexin 1α (n1α) and its role as synaptic organizer.
Marjorie Zambonino1 and Pamela Pereira2
Available from: http://dx.doi.org/10.21931/RB/2019.04.02.12
ABSTRACT
α – and b-neurexins (NRXNs) are transmembrane adhesion protein complexes localized in presynaptic membranes into neurons and interact with the postsynaptic neuroligins (NLGNs). Our findings indicate that the neurexin 1α (n1α) is a synaptic organizer that directs postsynaptic development in neurons, evidenced in GABAergic neurons and trials with Knock-out Mice. Also, the interactions between hypervariable surfaces of n1α and ligands (neurexophilin, a-dystroglycan, and GABAA) promotes a proper protein-binding recognition, and consequently, a better synaptic adhesion.
There is a direct relationship between mental disorders and the n1α assemblage because NRXN1 gene encodes for n1α proteins which are involved in the transmission of information into the brain. For this reason, damage in this complex-protein or some neurexin gene variations causes pathological abnormalities and neuropsychiatric diseases such as schizophrenia, autism spectrum disorders, and intellectual disabilities.
Keywords: neurexin 1α, transmembrane adhesion protein, neuropsychiatric alterations, Knock-out mice, synaptic adhesion.
INTRODUCTION
The molecular dynamic of neurons consists of projections between several postsynaptic partners trough different connections. In neural circuits, the structure of n1α carries out connectivity through a synaptic adhesion and directs the neural postsynaptic development such as the interconnection of GABAergic neurons (neurons that use GABA as its neurotransmitter)1.
N1α are a type of synaptic organizers anchored mostly to the pre-synaptic membranes that promote synapse formation through signaling and trans-synaptic adhesion.2 The global organization of n1α is a combination of flexible and rigid binding domains, which composition promote the allosteric regulation of protein partner binding and the synaptic cleft. The singular shape of n1α is produced by interplay the structural elements within modules of laminin, neurexin or globulin, and with other postsynaptic arrangements like NLGNs.3
On the other hand, diseases linked to the malfunction of the n1α have a broad spectrum. This protein-complex is directly linked to the synapses of neurons and the subsequent processing of information into neural circuits.4 This review will explain the architecture and the functioning of the n1α, and likewise, its impact both in synapsis organization and in the development of some types of pathologies such as autism and schizophrenia. As a result of any malfunction, damage, mutation or deletion in the NRXN1 gene.
Definition and structure of Neurexin 1α
Neurexins are important synaptic cell adhesion molecules (CAMs) from a family of mostly presynaptic adhesion proteins involved in neuronal networks.5 They form trans-synaptic complexes with postsynaptic neuroligins and other binding partners in the synaptic cleft such as endogenous ligands including LRRTM family members (a transmembrane protein that leads presynaptic differentiation in contacting axons), neurexophilin (neurexin-binding proteins), a-dystroglycan and GABAA receptors2. The extracellular domain consists of three neurexin repeats (I, II and III) containing the LNS-EGF-LNS modules. Three EGF-like repeats intersperse the six LNS domains, and six splice inserts SS1-SS6 which interact with proteins as the Figure 1A shows and the cytoplasmic tails that interact with exocytotic machinery.2,5
The n1α is expressed by the NRXN1 gene located on chromosome 2p16.3 that spans 1.12 Mb and contains 23 exons, being one of the most significant known human gene.6 It is exposed to relatively frequent damage including mutations, missense changes, translocation, complete gene deletion, and intragenic copy number alterations.7
Moreover, they contain an O-linked carbohydrate attachment sequence and a cysteine loop domain. Therefore, α NRNXs are diversified synaptic organizers with alternative mRNA splicing, which allows the formation of the ‘‘transsynaptic dialog’’ and the synapse maturation for a transmission efficiency6,8.
To solve the structure of n1α, an X-ray crystallography of bovine neurexin is used to obtain a resolution of 2.65 A° and n1a+SS3 to 2.95 A°. The crystal structure reveals an L-shaped molecule separated in repeats I, II and III. The first one is flexible due to the L1 and EGF-A are nonvisible; the second repeat is a horseshoe-shaped L3-EGF-B-L4 globular structure and repeats III adopts an L5-EGF-C-L6 extensive configuration. Furthermore, they contain a hypervariable surface with loops conformed by a Ca2+-binding site and splice inserts SS2, SS3, and SS4. 3,8 (Figure 1 C)
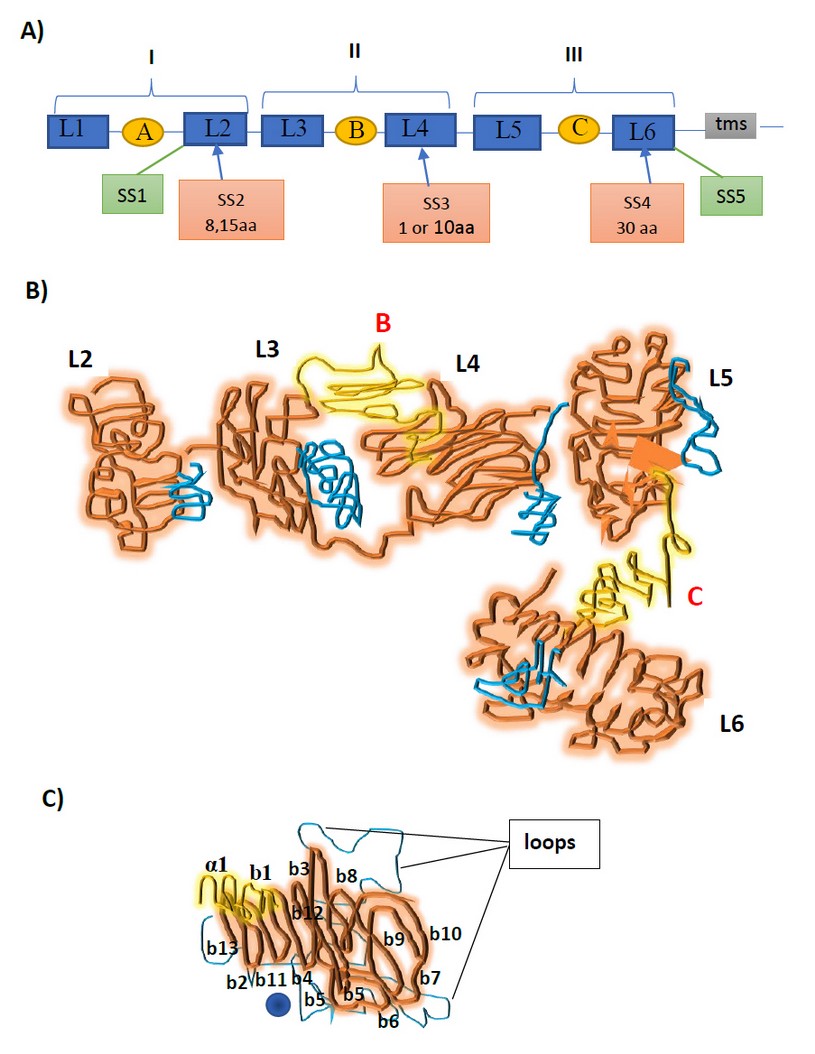
Figure 1. A) N1α is composed of six LNS or LG domains (blue squares) interspersed by three EGF like repeats (yellow ovals A, B, C). L6 is linked to a transmembrane segment (tms). Neurexin repeats I, II and III contains the splice inserts. B) A ribbon diagram of bovine n1a with domains L2–L6. The yellow compartments are b strands and α helices; loop b11-b12 is shown in blue, and the EGF-like repeats are in orange. There are imperceptible N-linked oligosaccharide chains attached to the complex. C) The n1α complex with b-loops and the ‘‘hypervariable surface’’ that carries a Ca2+-binding site (blue sphere).
Synaptic functioning
Due to the hypervariable surfaces of L2, L3, L4, L5, and L6 are putative binding sites of one side of the molecule, interacting ligands would assemble on just one side of the extracellular domain. This characteristic facilitates presynaptically tethered n1α to select multiple conventions of protein-partner recognition.9 (Figure 1B)
Spatial n1α architectures suggest a sizeable macromolecular complex in the synaptic cleft, where protein partners interact with two LNS domains. By this way, they are capable of acting in a cell-specific way. One evidence is the synapses formed between GABAergic-cholinergic motor neurons of the motor circuit of nematode Caenorhabditis elegans and the body wall muscles.1 The n1α directs the outgrowth of dendritic spine-like structures due to post-synaptic morphological development, but it is not necessary for synaptic connectivity with muscles.
Furthermore, the assemblage of cascades of synapses in overlapping neural circuits is significant for an organism’s survival, by integrating and processing sensory inputs to motor outputs. The communication between neurons via synapses is essential for the transformation of information and cognitive performance in the brain.10 N1α is also required for calcium-triggered neurotransmitter release and the correct working of voltage-gated calcium channels in the synapses of the brainstem and neocortex.19
Technics for studying n1α:
To understand the synaptic protein network, the levels of α neurexins and their ligands have to be manipulated in animal models. In the first place, the methods to analyze the conformational structure and flexibility of n1α, are based on the configuration of the LNS domains, L2 and L6 (which contain protein binding sites):
– Individual particle electron tomography (IPET), allows the 3D reconstruction of 110 n1α protein molecules L1-L6 at ~15 Å. These individual particles are large, multi-domain synaptic organizers like contactin-associated, protein-like-2 (CNTNAP2) and calsyntenin-3 (CLSTN3)1.– X-ray crystallography to analyze the structure of n1 α L2-L3 to 2.8 Å.– SAXS, to determine the impact of splice insert SS6 on the flexibility of n1 α repeat III (L5-EgfC-L6), and consequently in neuronal activity.
This kind of methods determines the allosteric control and protein partner biding of n1 α architecture platform. On the other hand, scientist do some experiments with a knock out rats using techniques of molecular biology. This method of knockout (KO) animals is useful to demonstrate the essential role of the NRXN genes in synaptic transmission, extracellular synaptic interactions and the regulation of N- and P/Q-type Ca2+ channels.11
They produce a heterozygous partial deletion in the n1α gene (NRXN1, 2p16.3) and make comparisons between the behavior of knock out individuals and normal ones, to show that alterations in n1α cause ASD.12 The behavioral testing of mice consists of multiple deletions of the a-isoforms of NRXN to analyze the phenotypes of relevance to human disorders. Mice have exposed to anxiety, cognitive, social and buried food tasks, which alterations are symptoms of autism in some human patients. The investigators perform three trials with an open field, light/dark box, and elevated plus maze in both male and female individuals.10,11
The results of genotype-phenotype correlation present in Nrxn1α KO Mice (with the absence of NRNX1), shows that knock out mice present some behavioral symptoms highly associated with ASD.12 Also, the male population suffer more of reduced locomotor activity, reduced nest building, abnormal pattern of spatial memory and an increase of anxiety/altered social behaviors like aggression, social impairment and inflexibility/stereotype.12,13
Neuropsychiatric disorders
The dysfunction of n1α protein and alterations in genes encoding neurexins or neuroligins cause variations in excitatory and inhibitory transmission. The hemizygous exonic deletions within the NRNX 1 gene is involved in neuropsychiatric diseases and cognitive illnesses such as intellectual disability, developmental delay, autism spectrum disorder (ASD) and schizophrenia (SZ).8 For the proper study in human, mammals contain three neurexin genes (NRXN1-3), which encodes for two isoforms, an extracellularly longer α -neurexins and shorter b-neurexins.3
Schizophrenia
Schizophrenia is a severe mental illness that affects the perception of the individual by altering the reality14 directly. There are some critical factors that affect the correct function of n1α: the copy number variations, the single nucleotide polymorphism (SNP), point mutations and gene deletions.4
The development of this disease is due to interactions between n1α and the isoforms of neuroligin and neurexin-attach organic compounds that form neurexophilins. This complex is involved in the mechanism of the differential electric potential of calcium channels, which is essential in the synaptic transmission of information on the brain.15 On the other hand, the chromosomal copy number variations (CNV) include genes involved mainly in sensory perception. Therefore, any n1α gene deletion affects neurocognitive skills/deficits and cause psychiatric disorders.4
Autism
Autism spectrum disorder (ASD) covers many symptomatologies and phenotypic expression that make social interactions difficult.16 One strong evidence is the missense mutations and deletions in exons which produce abnormal n1α. Besides, scientific studies showed that CNVs in n1α are associated with cognitive capability alterations and language development disorders related to autism17
In this sense, CNVs are a vital source of genetic variation resulted in phenotypic diversity and evolution; but its high amount leads a copy number polymorphism (an indicator of genomic instability) and pathogenesis like cancer disease. Experimental trials with computational programs demonstrate that many mutations and abnormal frequencies in CNVs, during transmission between generations, change the secondary structure and the functionality of n1α protein, and consequently, produce ASD. 4
Intellectual disabilities
Intellectual disability is a term used when a person has some limitations in their brain functioning. This illness prevents a healthy development in communication and social skills because it produces a slow learning process and a minor intellectual coefficient.18 The leading cause is a deletion of a part of the gene that codifies for n1α protein, making difficult the transmission of information into the brain.19,20

Table 1: (Pereira J. Some diseases related to variations of neurexin1α. Ibarra; 2018). The table shows 3 of the most severe neurological illness related to the variations into neurexin1α. We can see that (CNV) is the constant factor that concern to the majority of neurological illness linked to variations of NRXN1α.
Schizophrenia
Schizophrenia is a severe mental illness that affects the perception of the individual by altering the reality14 directly. There are some critical factors that affect the correct function of n1α: the copy number variations, the single nucleotide polymorphism (SNP), point mutations and gene deletions.4
The development of this disease is due to interactions between n1α and the isoforms of neuroligin and neurexin-attach organic compounds that form neurexophilins. This complex is involved in the mechanism of the differential electric potential of calcium channels, which is essential in the synaptic transmission of information on the brain.15 On the other hand, the chromosomal copy number variations (CNV) include genes involved mainly in sensory perception. Therefore, any n1α gene deletion affects neurocognitive skills/deficits and cause psychiatric disorders.4
Autism
Autism spectrum disorder (ASD) covers many symptomatologies and phenotypic expression that make social interactions difficult.16 One strong evidence is the missense mutations and deletions in exons which produce abnormal n1α. Besides, scientific studies showed that CNVs in n1α are associated with cognitive capability alterations and language development disorders related to autism17
In this sense, CNVs are a vital source of genetic variation resulted in phenotypic diversity and evolution; but its high amount leads a copy number polymorphism (an indicator of genomic instability) and pathogenesis like cancer disease. Experimental trials with computational programs demonstrate that many mutations and abnormal frequencies in CNVs, during transmission between generations, change the secondary structure and the functionality of n1α protein, and consequently, produce ASD. 4
Intellectual disabilities
Intellectual disability is a term used when a person has some limitations in their brain functioning. This illness prevents a healthy development in communication and social skills because it produces a slow learning process and a minor intellectual coefficient.18 The leading cause is a deletion of a part of the gene that codifies for n1α protein, making difficult the transmission of information into the brain.19,20
CONCLUSIONS
Neurexins 1α is essential transmembrane cell-adhesion proteins that modulate synaptic activity in the central and peripheral nervous system. The n1α architecture influences the transmission of information on the brain and the proper recruitment and organization of n1α protein partners. This is essential for better performance in regulation and synaptic transmission between neural circuits.
NRXNs damage represents a synaptic failure that affects immediately their proper releasing of full potential and contributes to a broad spectrum of neuropsychiatric disorders such as autism and schizophrenia. The variation in CVG that encode for α-NRXN1 is the most critical factor because of duplications, deletions or mutations in the gene that can be catastrophic for the individual.
There are some new methods in the molecular biology field to study the conformation and the functionality of the neurexin 1α, such as the tomography IPET, X-ray crystallography, SAXS, and knockout animals. The last one (KO), has allowed the modification of the genome of rats in order to make advances in studies of pathologies that affect the human’s neuronal activity directly, and help to perform more reliable clinical trials to improve the quality of patients.
REFERENCES
1. Philbrook A, Ramachandran S, Lambert C, Oliver D, Florman J, Alkema M et al. Neurexin directs partner-specific synaptic connectivity in C. elegans. Neuroscience. 2018;5.
2. Liu J, Misra A, Reddy M, White M, Ren G, Rudenko G. Structural Plasticity of Neurexin 1α: Implications for its Role as Synaptic Organizer. Journal of Molecular Biology. 2018;430(21):4325-4343.
3. Miller M, Mileni M, Comoletti D, Stevens R, Harel M, Taylor P. The Crystal Structure of the α-Neurexin-1 Extracellular Region Reveals a Hinge Point for Mediating Synaptic Adhesion and Function. Structure. 2011;19(6):767-778.
4. Wang, K. (2017). The influence of neurexin 1 gene variants on cognitive ability in multiplex schizophrenia families. 1st ed. Tunku Abdul Rahman, p.133.
5. Chen F, Venugopal V, Murray B, Rudenko G. The Structure of Neurexin 1α Reveals Features Promoting a Role as Synaptic Organizer. Structure. 2011;19(6):779-789.
6. Jenkins A, Paterson C, Wang Y, Hyde T, Kleinman J, Law A. Neurexin 1 (NRXN1) splice isoform expression during human neocortical development and aging. Molecular Psychiatry. 2015;21(5):701-706.
7. Ching M, Shen Y, Tan W, Jeste S, Morrow E, Chen X et al. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2010;:n/a-n/a.
8. Chen L, Jiang M, Zhang B, Gokce O, Südhof T. Conditional Deletion of All Neurexins Defines Diversity of Essential Synaptic Organizer Functions for Neurexins. Neuron Article. 2017.
9. Rabaneda L, Robles-Lanuza E, Nieto-González J, Scholl F. Neurexin Dysfunction in Adult Neurons Results in Autistic-like Behavior in Mice. Cell Reports. 2014;8(2):338-346..
10. Neuroligins and neurexins link synaptic function to cognitive disease. Nature [Internet]. 2008 [cited 25 November 2018];455:903-911. Available from: https://www.nature.com/articles/nature07456
11. Grayton H, Missler M, Collier D, Fernandes C. Altered Social Behaviours in Neurexin 1α Knockout Mice Resemble Core Symptoms in Neurodevelopmental Disorders. PLoS ONE. 2013;8(6):e67114.
12. Esclassan F, Francois J, Phillips K, Loomis S, Gilmour G. Phenotypic Characterization of Nonsocial Behavioral Impairment in Neurexin 1 Knockout Rats. Behavioral Neuroscience. 2014;Volumen 129(1):12.
13. Laarakker M, Reinders N, Bruining H, Ophoff R, Kas M. Sex-Dependent Novelty Response in Neurexin-1α Mutant Mice. PLoS ONE. 2012;7(2):e31503.
14. National Institute of Mental Health Office of Science Policy, Planning, and Communications Science Writing, Press, and Dissemination Branch. SCHIZOPRENIA. United States; p. 6.
15. Borna G, Breuer D, Wang S, Rohlmann A, Coulon P, Vakili P. Modulation of synaptic function through the α-neurexin–specific ligand neurexophilin-1. aInstitute of Anatomy and Molecular Neurobiology, Westfälische Wilhelms-University. 2014;10.
16. U.S. Department of Health and Human Services National Institutes of Health National Institute of Mental Health. Autism Spectrum Disorder. United States; 2015 p. 8.
17. Enas K, Taiga K, Katsuhiko T. Neurexins and neuropsychiatric disorders. 1st ed. Shinshu, Japan; 2017.
18. Lujambio A, Sáenz A, Nava L, Piña C, Escobar M, etc al. Discapacidad intelectual. Mexico D.F; 2010 p. 59.
19. Supaporn Y, Oradawan P, Thanya S, Rawiwan R, Tippawan H. Mutation Screening of the Neurexin 1 Gene in Thai Patients with Intellectual Disability and Autism Spectrum Disorder. GENETIC TESTING AND MOLECULAR BIOMARKERS. 2014; Volume 18:6.
20. Sudhof T. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Leading Edge Review. 2017;25.
Received: 9 April 2019
Accepted: 11 May 2019
Marjorie Zambonino1 and Pamela Pereira2
Universidad de Investigación de Tecnología Experimental Yachay Tech
1,2School of Biological and Applied – Biomedical Engineering Department.
https://orcid.org/0000-0001-8519-02261, https://orcid.org/0000-0002-0708-54792



















