Vol 9 No 3 2024-1
Semejanza a fármacos in silico y análisis ADME de isoflavonas fitoestrogénicas para aplicaciones alimentarias
In silico druglikeness and ADME analysis of phytoestrogenic isoflavones for food applications
Liyoesmin Salinas-Rojas 1*, Arianna Rubio2, Luisa Matos3, Maria Isabel Lantero 4, Enrique Molina5.
1Faculty of Applied Sciences (University of Camagüey Ignacio Agramonte Loynaz / Camagüey / Cuba). ORCID 0000-0001-5405-3237
2Informatics (Center for Genetic Engineering and Biotechnology / Camagüey / Cuba); arianna.rubio@cigb.edu.cu. ORCID 0000-0002-4505-3945
3Faculty of Applied Sciences (University of Camagüey Ignacio Agramonte Loynaz / Camagüey / Cuba); luisa.matos@reduc.edu.cu. ORCID 0000-0002-2387-163X
4Institute of Pharmacy and Food (University of Havana / Havana / Cuba); mailantero@gmail.com.
5 Faculty of Applied Sciences (University of Camagüey Ignacio Agramonte Loynaz / Camagüey / Cuba); enrique.molina@reduc.edu.cu. ORCID 0000-0001-7987-1893
*Correspondence: salinasliyoesmin@gmail.com
DOI: 10.70373/RB/2024.09.03.1
Abstract
Isoflavones are bioactive compounds found in vegetables, particularly legumes, with potential nutraceutical properties. Their estrogenic activity, attributed to structural similarity to estradiol, allows them to bind and modulate estrogen receptors, conferring beneficial effects such as cancer prevention and treatment, osteoporosis, sarcopenia, and diabetes. However, their bioactivity and nutraceutical potential are limited by bioavailability. This study analyzed the pharmacokinetic properties, druglikeness, and bioavailability of 13 isoflavones using the web tool SwissADME. Results showed that none of the isoflavones fell completely within the optimal physicochemical properties for oral bioavailability, primarily due to their characteristic unsaturation. Puerarin exhibited high polarity and violated several druglikeness criteria, suggesting potential issues with absorption and permeability. The remaining compounds complied satisfactorily with druglikeness rules. Most isoflavones exhibited high intestinal membrane permeability, with neobavaisoflavone, formononetin, and daidzein also able to cross the blood-brain barrier. None acted as substrates for P-glycoprotein, and they generally worked as inhibitors of cytochromes CYP1A2, CYP2D6, and CYP3A4. These findings provide valuable insights into the pharmacokinetic behavior and druglikeness of isoflavones, aiding in the development of nutraceutical products and guiding further research on their bioavailability and biological effects.
Keywords
Isoflavones; bioavailability; pharmacokinetics; druglikeness; SwissADME; nutraceuticals; estrogenic activity
Introduction
The nutraceutical properties of phytoestrogens are of great interest primarily because of their potential ability to alleviate symptoms associated with menopause. These compounds are widely used as alternatives to hormone-replacement therapies. Among these bioactive compounds, isoflavones are particularly noteworthy (1).
Their estrogenic activity is attributed to their structural similarity to estradiol, the primary endogenous estrogen in mammals. They can bind to and modulate the activity of estrogen receptors alpha and beta, with the latter being recognized for its anticancer activity. Most isoflavones preferentially bind to beta-estrogen receptors (2). Estrogen receptors can regulate biological processes through different mechanisms. In genomics, ligands bind to estrogen receptors in the nucleus and generate conformational changes that dissociate chaperones, enabling dimerization and activation of target gene transcription. Non-classical regulation of gene expression involves protein-protein interactions of the receptor with other transcription factors, such as AP-1, and stimulation proteins (SP-1) with response elements (3). The beneficial effects associated with their consumption include cancer prevention and treatment (4,5), osteoporosis (6), sarcopenia (7), and diabetes (8).
Vegetables, particularly legumes, such as soybeans, beans, and alfalfa, are rich sources of isoflavones. The foods that contribute the most to isoflavone intake include soy-derived products, soybeans, soy flour, soy flakes, soy beverages, and fermented soy products such as miso and tempeh, among others. Some of the most promising compounds within this group are daidzein, genistein, glycitein, biochanin A and formononetin (9). Owing to their beneficial effects, isoflavones are now used in various commercial products such as functional health supplements, cosmetics, and beverages (2).
The bioactivity and nutraceutical potential of these compounds are limited by their bioavailability (10). In silico analyses can help predict behavior and reduce cost and time consumption. SwissADME is a web tool that can assist with this regard (11). The objective of this study was to analyze the pharmacokinetic properties, druglikeness, and bioavailability of 13 isoflavones using SwissADME.
Materials and methods
The SMILES (Simplified Molecular Input Line Entry System) strings of the studied isoflavones were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Table 1 provides detailed information including the compound name, database identifier, and SMILES.
Table 1. Smile codes of the 13 isoflavones evaluated in their bioavailability, druglikeness and pharmacokinetics profile using SwissAdme web platform services.
| Compound | Pubchem CID | Canonical SMILES |
| Genistein | 5280961 | C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O |
| Daidzein | 5281708 | C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3)O)O |
| Biochanin A | 5280373 | COC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)O |
| Tectorigenin | 5281811 | COC1=C(C2=C(C=C1O)OC=C(C2=O)C3=CC=C(C=C3)O)O |
| Puerarin | 53850774 | C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3C4C(C(C(C(O4)CO)O)O)O)O)O |
| 3′,4′,7-Trihydroxyisoflavone | 5284648 | C1=CC(=C(C=C1C2=COC3=C(C2=O)C=CC(=C3)O)O)O |
| Barpisoflavone A | 9944143 | COC1=CC(=CC2=C1C(=O)C(=CO2)C3=C(C=C(C=C3)O)O)O |
| 2′-Hydroxygenistein | 5282074 | C1=CC(=C(C=C1O)O)C2=COC3=CC(=CC(=C3C2=O)O)O |
| 5-O-Methylgenistein | 5748551 | COC1=C2C(=O)C(=COC2=CC(O)=C1)C1=CC=C(O)C=C1 |
| Gerontoisoflavone A | 15223506 | COC1=C(O)C=CC(=C1)C1=COC2=CC(O)=CC(OC)=C2C1=O |
| Calycosin | 5280448 | COC1=C(C=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)O)O |
| Formononetin | 5280378 | COC1=CC=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)O |
| Neobavaisoflavone | 5320053 | CC(=CCC1=C(C=CC(=C1)C2=COC3=C(C2=O)C=CC(=C3)O)O)C |
Using SMILES strings, in silico analysis was conducted on the freely accessible web platform SwissAdme (http://www.swissadme.ch/).
Results
Physicochemical properties and bioavailability
Bioavailability of a bioactive compound is influenced by its chemical and physical properties. Figure 1 shows the chemical structures of the studied isoflavones and the corresponding radar graph of bioavailability is shown below. In these graphs, the shaded pink area indicates the optimal physicochemical properties for optimal oral bioavailability.
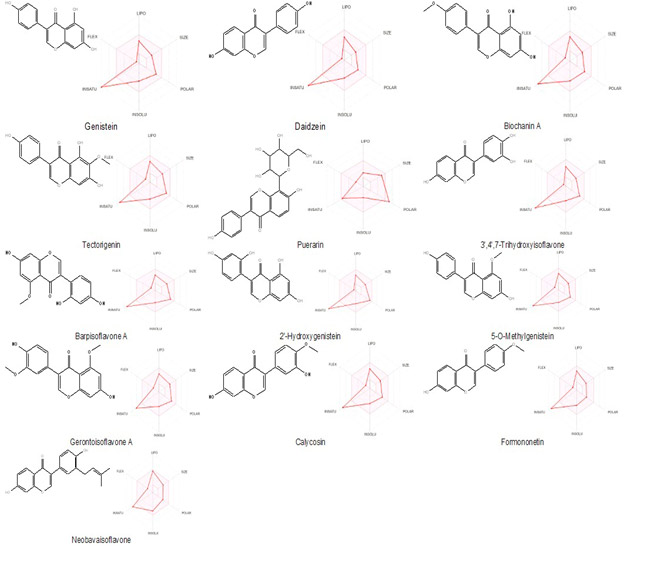 Figure 1. Chemical structures and bioavailability radars of isoflavones (lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility: no more than 9 rotatable bonds). The coordinate values are defined in (11).
Figure 1. Chemical structures and bioavailability radars of isoflavones (lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility: no more than 9 rotatable bonds). The coordinate values are defined in (11).
Analysis of the graphs shows that none of the isoflavones fall completely within the shaded pink area, which is due to the characteristic unsaturation of their structures. As shown in Table 2, the predicted values of the sp3 carbon fraction are less than the required 0.25, except for puerarin, whose corresponding value is 0.29. This compound also exhibited high polarity, given by a topological surface area of 160 Ų. Despite these results, a bioavailability score of 0.55 is predicted in all cases.
Table 2. The physicochemical properties of isoflavones were calculated using SwissADME.
| Compound | M.W1 (g/mol) | nHA2 | nAHA3 | F. Csp3 4 | RB5 | nHBA6 | nHBD7 | MR8 | TPSA (Å2)9 |
| Genistein | 270.24 | 20 | 16 | 0.00 | 1 | 5 | 3 | 73.9 | 90.90 |
| Daidzein | 254.24 | 19 | 16 | 0.00 | 1 | 4 | 2 | 71.97 | 70.67 |
| Biochanin A | 284.26 | 21 | 16 | 0.06 | 2 | 5 | 2 | 78.46 | 79.90 |
| Tectorigenin | 300.26 | 22 | 16 | 0.06 | 2 | 6 | 3 | 80.48 | 100.13 |
| Puerarin | 416.38 | 30 | 16 | 0.29 | 3 | 9 | 6 | 104.59 | 160.82 |
| 3′,4′,7-Trihydroxyisoflavone | 270.24 | 20 | 16 | 0.00 | 1 | 5 | 3 | 73.99 | 90.90 |
| Barpisoflavone A | 300.26 | 22 | 16 | 0.06 | 2 | 6 | 3 | 80.48 | 100.13 |
| 2′-Hydroxygenistein | 286.24 | 21 | 16 | 0.00 | 1 | 6 | 4 | 76.01 | 111.13 |
| 5-O-Methylgenistein | 284.26 | 21 | 16 | 0.06 | 2 | 5 | 2 | 78.46 | 79.90 |
| Gerontoisoflavone A | 314.29 | 23 | 16 | 0.12 | 3 | 6 | 2 | 84.95 | 89.13 |
| Calycosin | 284.26 | 21 | 16 | 0.06 | 2 | 5 | 2 | 78.46 | 79.90 |
| Formononetin | 268.26 | 20 | 16 | 0.06 | 2 | 4 | 1 | 76.43 | 59.67 |
| Neobavaisoflavone | 322.35 | 24 | 16 | 0.15 | 3 | 4 | 2 | 95.69 | 70.67 |
1M.W: Molecular weight; 2 nHA: No. heavy atom; 3nAHA: No. aromatic heavy atom; 4F. Csp3: No. of sp3 hybridized carbon out of total carbon count; 5RB: Rotatable bonds; 6nHBA: No. H-bond acceptors; 7nHBD: No. H-bond donors; 8MR: Molar refractivity; 9TPSA: Topological Polar Surface Area.
Druglikeness
The analyzed isoflavones pass druglikeness evaluation whith five tools: Lipinski, Muegge, Ghose, Veber and Egan (Table 3). Puerarin (except for Ghose) deviates owing to its elevated hydrogen bond donors and TPSA.
Table 3. Druglikeness of isoflavones.
| Compound | Lipinski | Ghose | Veber | Egan | Muegge |
| Genistein | Yes | Yes | Yes | Yes | Yes |
| Daidzein | Yes | Yes | Yes | Yes | Yes |
| Biochanin A | Yes | Yes | Yes | Yes | Yes |
| Tectorigenin | Yes | Yes | Yes | Yes | Yes |
| Puerarin | Violation
OH>5 |
Yes | Violation
TPSA>140 |
Violation
TPSA>131.6 |
Violation
TPSA>150 H-don>5 |
| 3′,4′,7-Trihydroxyisoflavone | Yes | Yes | Yes | Yes | Yes |
| Barpisoflavone A | Yes | Yes | Yes | Yes | Yes |
| 2′-Hydroxygenistein | Yes | Yes | Yes | Yes | Yes |
| 5-O-Methylgenistein | Yes | Yes | Yes | Yes | Yes |
| Gerontoisoflavone A | Yes | Yes | Yes | Yes | Yes |
| Calycosin | Yes | Yes | Yes | Yes | Yes |
| Formononetin | Yes | Yes | Yes | Yes | Yes |
| Neobavaisoflavone | Yes | Yes | Yes | Yes | Yes |
Pharmacokinetic profile
As shown in Figure 2, the isoflavones included in this study exhibited high permeability of the intestinal membrane, except for puerarin. Only neobavaisoflavone, formononetin, and daidzein, in addition to high intestinal absorption (HIA), can cross the blood-brain barrier (BBB)., can cross the blood-brain barrier.
Other relevant pharmacokinetic parameters are summarized in Table 4. It is important to note that none of these phytoestrogens act as substrates for P-glycoprotein (PGP). Generally, they work as inhibitors of cytochrome CYP1A2, CYP2D6, and CYP3A4.
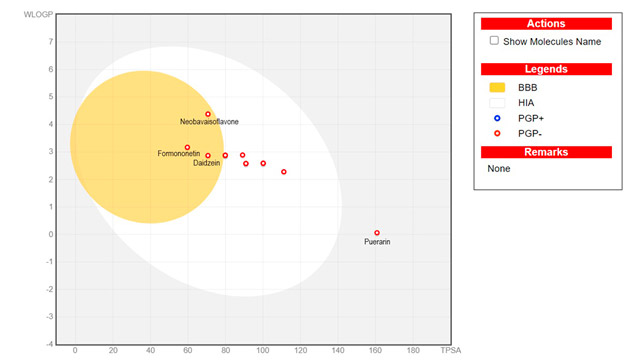 Figure 2. Boiled egg graph representations of isoflavones.
Figure 2. Boiled egg graph representations of isoflavones.
Table 4. Pharmacokinetic profile of isoflavones.
| Compound | GI abs. | P-gp subs. | Inhibitor | |||||
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | ||||
| Genistein | High | No | Yes | No | No | Yes | Yes | |
| Daidzein | High | No | Yes | No | No | Yes | Yes | |
| Biochanin A | High | No | Yes | No | No | Yes | Yes | |
| Tectorigenin | High | No | Yes | No | No | Yes | Yes | |
| Puerarin | Low | No | No | No | No | No | No | |
| 3′,4′,7-Trihydroxyisoflavone | High | No | Yes | No | No | Yes | Yes | |
| Barpisoflavone A | High | No | Yes | No | No | Yes | Yes | |
| 2′-Hydroxygenistein | High | No | Yes | No | No | Yes | Yes | |
| 5-O-Methylgenistein | High | No | Yes | No | No | Yes | Yes | |
| Gerontoisoflavone A | High | No | Yes | No | Yes | Yes | Yes | |
| Calycosin | High | No | Yes | No | No | Yes | Yes | |
| Formononetin | High | No | Yes | No | No | Yes | Yes | |
| Neobavaisoflavone | High | No | Yes | No | Yes | No | Yes | |
Discussion
Lipinski established the importance of physicochemical properties of a compound for ADME. He proposed five well-recognized and widely used rules to filter compound libraries, suggesting that violations of these rules could be associated with poor oral absorption and low permeability of the molecules (12). The characteristics of these rules are MW ≤ 500 Da, MLOGP ≤ 4.15, N or O ≤ 10, and NH or OH ≤ 5 (11). Puerarin, in its structure, has six hydroxyl groups that act as hydrogen donors, thus violating one of these rules. There is a correlation between the number of hydrogen acceptors and donors and the TPSA, where an increase in these groups reduces permeability and, therefore, absorption. The remaining compounds complied satisfactorily with these criteria.
This isoflavone also showed violations according to the Veber, Egan, and Muegge criteria. Its TPSA was 160.82 Ų, exceeding the recommended values of less than 140 Ų, 131.6 Ų, and 150 Ų. This parameter is critical for the permeability through passive membrane transport. When TPSA exceeds 120 Ų, poor absorption occurs; for brain penetration, negative effects are observed when TPSA exceeds 90 Ų (12). In the case of Muegge, as with Lipinski, the number of hydrogen donors is also considered, resulting in two violations of puerarin. These findings suggest that puerarin has poor gastrointestinal absorption. This is a crucial aspect to consider given its intended use as a bioactive compound for incorporation into foods, which may negatively impact its subsequent biological activity.
None of the isoflavones were predicted to behave as substrates for P-glycoprotein, which acts as a biological barrier by expelling toxins and xenobiotics from the body. This is a key pharmacokinetic factor to consider because of its influence on absorption, which is decisive for the oral bioavailability. Its function limits absorption from the intestine into the systemic circulation during oral administration, restricts penetration into the brain, and facilitates excretion (13).
The biodisponibility radar showed that these compounds have an sp3 carbon fraction below the optimal lower range, set at 0.25. This factor was considered to be correlated with the solubility of the compounds. Higher saturation values give a molecule a greater chance of success during the discovery and use processes (14). Isoflavones are known for their low solubility in water, which can contribute to their reduced bioactivity (10).
Only puerarin exceeded the specified range for TPSA values. This is a critical aspect related to the ability of compounds to traverse biological membranes, such as those in the intestine and brain (11). This relationship is depicted in the boiled egg diagram, where the shaded white area corresponds to compounds with high gastrointestinal absorption and the yolk represents molecules capable of permeating the brain (15). As observed, only one phytoestrogen falls outside these areas.
The inhibition of cytochrome P-450 isoenzymes by the studied compounds is of interest, as it reveals which enzymes may be affected. This is significant because isoenzymes inhibition can lead to drug interactions and induce undesired effects (11).
Except for puerarin, the studied isoflavones have good absorption, distribution, metabolism, and excretion profiles. Abbott bioavailability score seeks to predicts the probability of a compound to have at least 10 % oral bioavailability in rat or measurable Caco-2 permeability which predicts probability of a compound to have F >10 % based on the predominant charge at biological pH in a rat model (11). All isoflavones exhibited a uniform and good bioavailability score of 0.55. These results coincide with previous ADME studies carried out on daidzein (16) and other compounds such as genistein, glycitein, formononetin and biochanin A (17). The prediction of an adequate ADME profile makes it possible to identify them as potential candidates to continue with subsequent studies until their effective incorporation as bioactives in functional foods. However, numerous studies have indicated that they have a low bioavailability in vivo. Factors such as the food matrix, age, and gender of consumers, as well as extensive biotransformation by the microbiota, play a role in this aspect (18). The last issue is gaining interest from the scientific community (19,20). Further studies should be conducted to clarify these issues.
Conclusions
The in silico analysis of ADME properties, with a focus on pharmacokinetics, druglikeness, and bioavailability, of 13 estrogenic isoflavones using the SwissAdme web tool predicted an adequate profile. The exception was puerarin, which had a high TPSA value and six hydroxyl groups acting as hydrogen donors and showed poor absorption. All compounds met the druglikeness criteria established by Lipinski, Veber, Egan, and Muegge. None of these compounds was predicted to act as substrates for P-glycoprotein. Adequate bioavailability with high gastrointestinal absorption is predicted, along with the ability of neobavaisoflavone, formononetin, and daidzein to permeate the blood-brain barrier. These results contribute to identifying the phytoestrogenic isoflavones studied with an adequate ADME profile as potential candidates for incorporation as bioactuve compounds in foods.
Supplementary Materials: The following are available online at www.revistabionatura.com/xxx/s1, Figure S1: title, Table S1: title, Video S1: title.
Author Contributions: Conceptualization, L.S. and E.M. and M.L.; methodology, L.S.; formal analysis, L.S.; investigation, L.S. and A.R.; data curation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, L.S. and L.M.; visualization, A.R.; supervision, E.M. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Data Availability Statements in the «Bionatura Research Data Policies» section at https://www.revistabionatura.com/policies.html.
Conflicts of Interest: The authors declare no conflict of interest.
Acknowledgments: We are grateful to the Swiss Institute of Bioinformatics for providing their web resources for free use.
References
- De Franciscis P, Colacurci N, Riemma G, Conte A, Pittana E, Guida M, et al. A Nutraceutical Approach to Menopausal Complaints. Medicina (B Aires). 2019 Aug 28;55(9):544. DOI 10.3390/medicina55090544
- Kim IS. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants. 2021 Jun 30;10(7):1064. DOI 10.3390/antiox10071064
- Cipolletti M, Solar Fernandez V, Montalesi E, Marino M, Fiocchetti M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ers) signaling. Int J Mol Sci [Internet]. 2018;19(9):2624. Available from: DOI:10.3390/ijms19092624
- Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, et al. Intake of Soy, Soy Isoflavones and Soy Protein and Risk of Cancer Incidence and Mortality. Front Nutr. 2022 Mar 4;9. DOI 10.3389/fnut.2022.847421
- Kaufman‐Szymczyk A, Jalmuzna J, Lubecka‐Gajewska K. Soy‐derived isoflavones as chemo‐preventive agents targeting multiple signalling pathways for cancer prevention and therapy. Br J Pharmacol. 2024 Mar 25. DOI 10.1111/bph.16353. Epub ahead of print.
- Akhlaghi M, Ghasemi Nasab M, Riasatian M, Sadeghi F. Soy isoflavones prevent bone resorption and loss, a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020 Aug 5;60(14):2327–41. DOI 10.1080/10408398.2019.1635078
- Lee SY, Yoo JI. Soybean isoflavones potentially prevent sarcopenia: a systematic review. Journal of Ethnic Foods. 2023 Dec 8;10(1):48. DOI 10.1186/s42779-023-00210-6
- Kuryłowicz A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int J Mol Sci. 2020 Dec 28;22(1):218. DOI 10.3390/ijms22010218
- Gómez-Zorita S, González-Arceo M, Fernández-Quintela A, Eseberri I, Trepiana J, Portillo MP. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients. 2020 Dec 17;12(12):3853. DOI 10.3390/nu12123853
- Liu H, Wang Y, Zhu D, Xu J, Xu X, Liu J. Bioaccessibility and Application of Soybean Isoflavones: A Review. Food Reviews International. 2023 Sep 8;39(8):5948–67. DOI 10.1080/87559129.2022.2103824
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017 Mar 3;7(1):42717. DOI 10.1038/srep42717
- Varma MVS, Obach RS, Rotter C, Miller HR, Chang G, Steyn SJ, et al. Physicochemical Space for Optimum Oral Bioavailability: Contribution of Human Intestinal Absorption and First-Pass Elimination. J Med Chem. 2010 Feb 11;53(3):1098–108. DOI 10.1021/jm901371v
- Elmeliegy M, Vourvahis M, Guo C, Wang DD. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug–Drug Interaction Studies. Clin Pharmacokinet. 2020 Jun 13;59(6):699–714. DOI 10.1007/s40262-020-00867-1
- Lovering F, Bikker J, Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J Med Chem. 2009 Nov 12;52(21):6752–6. DOI 10.1021/jm901241e
- Daina A, Zoete V. A BOILED‐Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 2016 Jun 6;11(11):1117–21. DOI 10.1002/cmdc.201600182
- Haseeba Sardar. Drug like potential of Daidzein using SwissADME prediction: In silico Approaches. PHYTONutrients. 2023 Nov 28;01–7. DOI 10.62368/pn.vi.19
- Muslikh FA, Samudra RR, Ma’arif B, Ulhaq ZS, Hardjono S, Agil M. In Silico Molecular Docking and ADMET Analysis for Drug Development of Phytoestrogens Compound with Its Evaluation of Neurodegenerative Diseases. Borneo Journal of Pharmacy. 2022 Nov 30;5(4):357–66. DOI 10.33084/bjop.v5i4.3801
- Turner NJ, Thomson BM, Shaw IC. Bioactive Isoflavones in Functional Foods: The Importance of Gut Microflora on Bioavailability. Nutr Rev. 2003 Jun 1;61(6):204–13. DOI 10.1301/nr.2003.jun.204-213
- Stojanov S, Kreft S. Gut Microbiota and the Metabolism of Phytoestrogens. Revista Brasileira de Farmacognosia. 2020 Apr 16;30(2):145–54. DOI 10.1007/s43450-020-00049-x
- Seyed Hameed AS, Rawat PS, Meng X, Liu W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol Adv. 2020 Nov;43:107576. DOI 10.1016/j.biotechadv.2020.107576
| Received: 15 June 2024 | Accepted: 28 August 2024 | Published: 14 September 2024 |
Citation: Salinas-Rojas, L., Rubio, A., Matos, L., Lantero, M., Molina, E., Semejanza a fármacos in silico y análisis ADME de isoflavonas fitoestrogénicas para aplicaciones alimentarias. Bionatura. 2024; Volume (9). No 3.
Peer review information: Bionatura thanks the anonymous reviewers for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are freely and permanently accessible online immediately after publication, without subscription charges or registration barriers.
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)



















