Vol 8 No 4 2023 – 57
Faisal Nasser Jaber 1, Aqeel Alyousuf 1*, and H. H. Al-Saffar2
1 Department of Plant Protection, College of Agriculture, University of Basrah, Iraq; faisal.nasser@uobasrah.edu.iq.
2 Department of Plant Protection, College of Agriculture, University of Barah, Iraq
3 Iraq Natural History Research Center and Museum, University of Baghdad, Iraq 2; dr.hanaa66@uobaghdad.edu.iq
* Correspondence: aqeel.alyousuf@okstate.edu
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.62
ABSTRACT
The study aimed to investigate the seasonal presence and characterization of the group of butterflies (abundance, richness and biodiversity) in the agroecosystem of Brassicaceae plants in the Basra province. Two different ecosystem sites (Karmat Ali at the sedimentary habitat and Al-Zubair region at the desert habitat) were sampled during the growing season of 2020/2021. There are 823 and 507 individuals at the Karmat Ali and Al-Zubair sites, respectively; the specimens consist of 9 butterflies belonging to four families. The most abundant family was Nymphalidae (60%), followed by Lycainidae (28%) and Pieridae (9%), while Hesperiidae recorded the lowest relative abundance of 3%. The highest butterfly population was recorded for Vanessa Carudi, while Pieris rapae had the lowest density. The results showed differences in abundance, species richness, and Diversity of butterflies at the sites. The highest values of Diversity and richness were at Karmat Ali, compared to the Al-Zubair region. The study of butterflies’ abundance and biodiversity indices indicated that environmental factors and the polyculture plantations support the butterfly population in agricultural habitats.
Keywords: Basra; Biodiversity; Brassicaceae; Butterflies; Shannon index; Richness.
INTRODUCTION
Butterflies are considered adequate ecological representatives for the invertebrates 1-4 and clear evidence of the changes in the environmental quality. Butterflies also play critical ecological roles in natural and agricultural habitats; they perform basic ecosystem services 5-7 by recycling essential nutrients (nitrogen, potassium and phosphorus) which are required by crops; adult butterflies usually suck the nectar, and larvae feed on the leaves of different plants of the agricultural landscapes, then releasing their feces containing some of these nutrients 8-11. Also, butterflies exist in the food chain of many vertebrates, such as birds and lizards 12, and they host many parasites, which decrease the pest populations 13, 14.
In the last few decades, the population of butterfly species has declined dramatically worldwide due to environmental changes and habitat destruction of 15. For example, the number of butterflies in the United States has decreased sharply due to global warming and environmental changes 16, 17; the population of the western monarch is declining at an estimated rate of 1.6% annually; and more than 450 species of butterflies, including the western monarch butterflies, have declined by 99.9% since the 1980s 18. A declining population of butterfly species was also observed in Europe 19; for example, 76% of the 59 species of migratory and endemic butterflies in the United Kingdom between 1976 and 2014 20. The development of ecological indices is essential in the field of biodiversity conservation. Butterflies quickly respond to ecosystem disturbance. Thus, they have been specified as significant biological indicators for evaluating the biodiversity of natural and agricultural habitats and monitoring the response of the ecosystems to environmental change and degradation21.
Globally, the decline in the number of butterfly species and habitat losses has led to an increased consciousness of conserving butterflies and their habitat 22. Due to the critical role of butterflies, which provide essential environmental services to crops and native wild plant species in many ecosystems, extensive research on the habitat requirement of butterflies is essential to promoting conservation efforts 23,24. Therefore, the conservation and the Diversity of butterfly species (especially the pollinators) requires a broad understanding of their foraging behavior and their spatio-temporal distribution in agricultural habitats 25; however, most specialized butterfly species usually depend on a limited set of host plants for their larval stage, or they use a limited number of nectar sources, even when a large number of nectar-producing plants are available 26, 27; butterflies also tend to use areas within the site where host plants are located near nectar sources or chose the host plants for laying eggs on the host plants close to the nectar sources; thus the correlation of egg laying and feeding on nectar must be studied according to the abundance of the host plants and nectar sources 28.
Despite the Diversity of butterflies in many natural habitats and their importance, especially concerning their ecological, behavioral, and functional role (especially pollination) 5, 8, little research has been done about the role of butterflies on the Biodiversity in Iraq 29-32. In agricultural ecosystems, many butterfly species play an essential role as pollinators of several crops that humans depend on for their livelihoods 33, 34; and no published data describe the Diversity of butterflies present in agricultural habitats in Iraq. The current study aimed to investigate the seasonal presence and characterization of the group of butterflies (abundance, richness and biodiversity) in the agroecosystem of Brassicaceae plants in the Basra province.
In the last few decades, the population of butterfly species has declined dramatically worldwide due to environmental changes and habitat destruction of 15. For example, the number of butterflies in the United States has decreased sharply due to global warming and environmental changes 16, 17; the population of the western monarch is declining at an estimated rate of 1.6% annually; and more than 450 species of butterflies, including the western monarch butterflies, have declined by 99.9% since the 1980s 18. A declining population of butterfly species was also observed in Europe 19; for example, 76% of the 59 species of migratory and endemic butterflies in the United Kingdom between 1976 and 2014 20. The development of ecological indices is essential in the field of biodiversity conservation. Butterflies quickly respond to ecosystem disturbance. Thus, they have been specified as significant biological indicators for evaluating the biodiversity of natural and agricultural habitats and monitoring the response of the ecosystems to environmental change and degradation21.
Globally, the decline in the number of butterfly species and habitat losses has led to an increased consciousness of conserving butterflies and their habitat 22. Due to the critical role of butterflies, which provide essential environmental services to crops and native wild plant species in many ecosystems, extensive research on the habitat requirement of butterflies is essential to promoting conservation efforts 23,24. Therefore, the conservation and the Diversity of butterfly species (especially the pollinators) requires a broad understanding of their foraging behavior and their spatio-temporal distribution in agricultural habitats 25; however, most specialized butterfly species usually depend on a limited set of host plants for their larval stage, or they use a limited number of nectar sources, even when a large number of nectar-producing plants are available 26, 27; butterflies also tend to use areas within the site where host plants are located near nectar sources or chose the host plants for laying eggs on the host plants close to the nectar sources; thus the correlation of egg laying and feeding on nectar must be studied according to the abundance of the host plants and nectar sources 28.
Despite the Diversity of butterflies in many natural habitats and their importance, especially concerning their ecological, behavioral, and functional role (especially pollination) 5, 8, little research has been done about the role of butterflies on the Biodiversity in Iraq 29-32. In agricultural ecosystems, many butterfly species play an essential role as pollinators of several crops that humans depend on for their livelihoods 33, 34; and no published data describe the Diversity of butterflies present in agricultural habitats in Iraq. The current study aimed to investigate the seasonal presence and characterization of the group of butterflies (abundance, richness and biodiversity) in the agroecosystem of Brassicaceae plants in the Basra province.
MATERIALS AND METHODS
Study Sites
Two sites were chosen to study butterflies’ seasonal activity and Biodiversity in Basra Province, southern Iraq, during the 2020-2021 growing season. The first site represented the agroecosystem of the sedimentary plain habitat 35, at the region of Karmat Ali; a field in agricultural station of the College of Agriculture at the University of Basra, close to Shatt Al-Arab river, was chosen as a permanent station No. 1 (30 25 45.1″ N, 47 55 52.1″ E), Where different crops are grown annually, in addition to the presence of trees such as date palm and Jujube. The second site represented the agroecosystem of the desert habitat 35, which was designated for the cultivation of the tomato crop and some leafy crops, mainly in Basra province; a field was chosen in Al-Zubair region as a permanent station No. 2 (30 39 5.47″ N, 47 42 34.32″ E). Each field (12 x 60 m2) was prepared, divided into plots, and planted with seven Brassicaceae crops (White radish Raphanus sativus subsp. acanthiformis, Arugula Eurica Sativa, rapeseed Brassica napus, mustard Brassica carinata, broccoli Brassica oleracea, variety italica, cauliflower Brassica oleracea, variety botrytis, cabbage Brassica oleracea, variety capitata).
Butterfly community composition and seasonal activity
During the butterfly flight season, Butterflies were monitored and collected for each species at each site biweekly during the sampling period of the growing season of 2020/2021 by using the insect net. Butterflies were sampled between 8:30 and 12:30 am and transferred to the laboratory for identification; specimens were kept in the insect collection box after hardening and recording all the necessary information. Butterfly species were diagnosed using taxonomic keys 36, 37. Butterfly Relative abundance was estimated for each species at both studied sites 38.
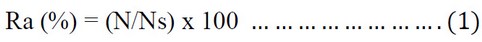
Where Ra= Relative abundance, N = the number of individuals of one species in the sample, Ns = the total number of individuals.
Biodiversity indices
Four ecological indices were used to measure the biodiversity of the butterfly community in the agroecosystems of Brassicaceae plants. The values of the biodiversity indices for the butterflies were calculated during the agricultural season from 1/11/2020 to 1/4/2021:
Shannon Diversity Index (H)
The diversity index values were calculated according to the equation mentioned in 39.
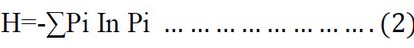
Where H = the value of Diversity, Pi = the ratio of the number of individuals of each species to the total number.
The Shannon-Wiener index value usually is between 1.5 and 3.5 for ecological data and rarely exceeds 4.0.
Richness index (D)
The value of the Richness index was calculated according to the equation given in 40:
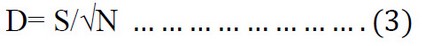
Where D = the value of Richness, S = number of species, N = total number of individuals in the sample
Evenness index (J)
The Evenness index was determined according to 41:
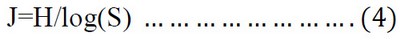
Where J = the Evenness value, H = value of the index of Diversity, S = number of species
Dominance index (d)
This index was calculated according to the equation in 42:
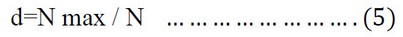
Since d = the value of Dominance, N max = number of individuals of the dominant species, N = total individuals in the sample.
The reciprocal of the index, 1/d, is often used so that an increase in the index’s value is accompanied by an increase in Diversity and a decrease in dominance.
Statistical analysis
The results of Butterflies-seasonal activity were analyzed according to ANOVA; then, the averages were compared using the least significant difference at a probability level 0.05.
RESULTS
Butterfly community composition and seasonal activity
The study of the community composition of butterflies showed that the total numbers of individuals collected were 823 and 507 individuals at the Karmat Ali and Al-Zubair sites, respectively; the specimens consist of 9 species of butterflies belonging to four families of the order Lepidoptera (Table1); the results of Table (2) indicated that there were significant differences (P < 0.05) among the butterflies populations associated with Brassicaceae plants; butterflies showed highest population of 4.71 individuals/ month at Karmat Ali, compared to 3.28 individuals/ month in the Al-Zubair region. However, the highest butterfly population was recorded for Vanessa crude, which reached 12.73 individuals/ month. At the same time, Belenois aurora had the lowest density, with an average of 0.2 individuals/ month during the growing season. V. carudi recorded the highest rate of 15.55 individuals/ month in the Karmat Ali site, while B. aurora had the lowest rate of 0.2 individuals/ month at both study sites.
Also, the results showed significant differences (P < 0.05) in the densities of butterfly species during the growing season in the two study stations (Table 3); the highest population of butterfly species was in March, with an average of 4.21 individuals/month, while the lowest density was found in January, reaching 3,065 individuals/month in both study sites. The highest rate of butterflies was recorded in the Karmat Ali area in March, with an average of 5.73 individuals/month, compared to the lowest number of 2.08 individuals in December. However, in the Al-Zubair area, the highest and lowest populations of 5.58 and 1.37 individuals were found in December and February, respectively.
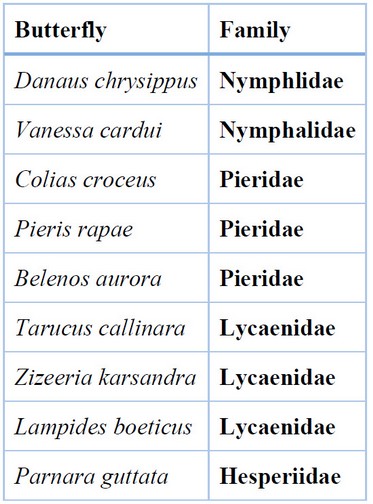
Table 1. Butterflies associated with Brassicaceae crops in Basra Province.
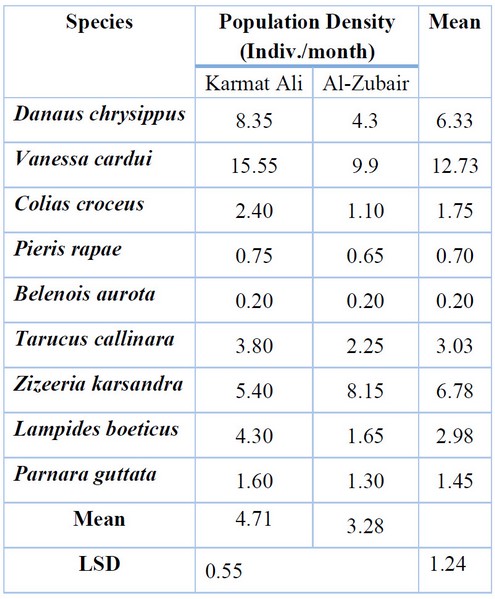
L.S.D. for interaction butterflies and regions (1.759).
Table 2. Population density of butterfly species in the two study sites during the growing season 2020-2021.
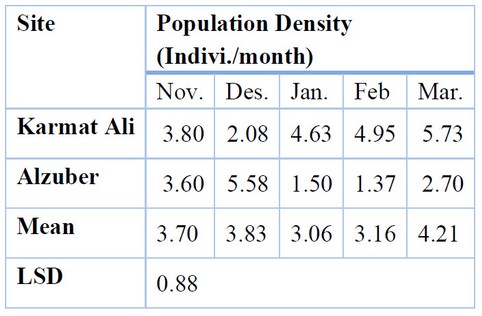
L.S.D. for interaction Months and sites (1.24).
Table 3. The seasonal activity of butterfly species in the two study areas 2020-2021.
The results of Table (4) showed that there were significant (P < 0.05) differences among the populations of butterfly species; the butterfly V. carudi was numerically superior during the growing season; the density was 16.88 individuals in March; however, the species Belenois aurota has not been recorded during the season except in November, with an average of 1.00 individuals/month.
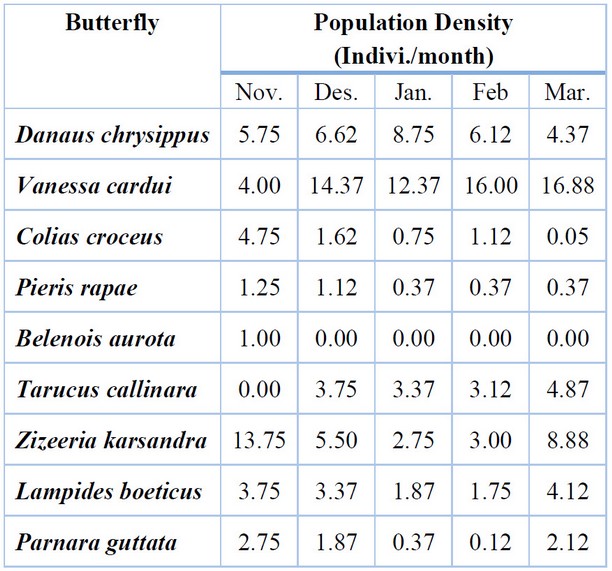
L.S.D. for interaction Butterflies and Months (2.78).
Table 4. The seasonal activity of butterfly species in Basra province during the growing season 2020-2021.
Relative abundance
The results of Table (5) showed that the highest relative abundance was recorded for the butterfly V. carudion the crops of the Brassicaceae during the growing season 2020-2021, at a rate of 38.72%, followed by the butterfly Danaus chrysippus, with the relative abundance of 16.76%; while the lowest relative abundance was recorded for the Pieris rapae, with an average of 1.05%. The results of the study also showed that the family Nymphalidae was the most abundant, reaching 60%, followed by Lycainidae (28%) and Pieridae (9%), while Hesperiidae recorded the lowest relative abundance of 3% (Figure 1).
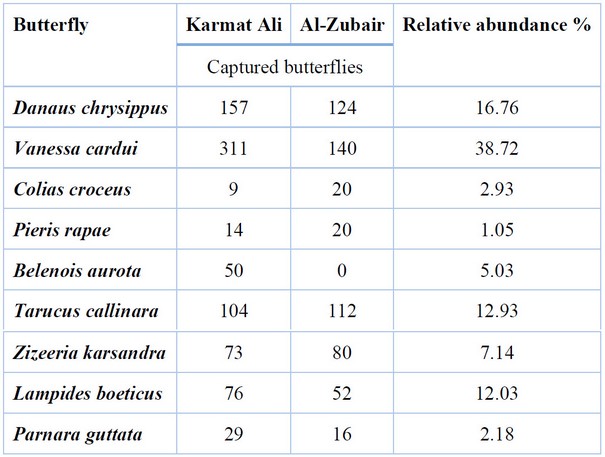
Table 5. The relative abundance of butterflies associated with the Brassicaceae crops in Basra during the growing season of 2020-2021.
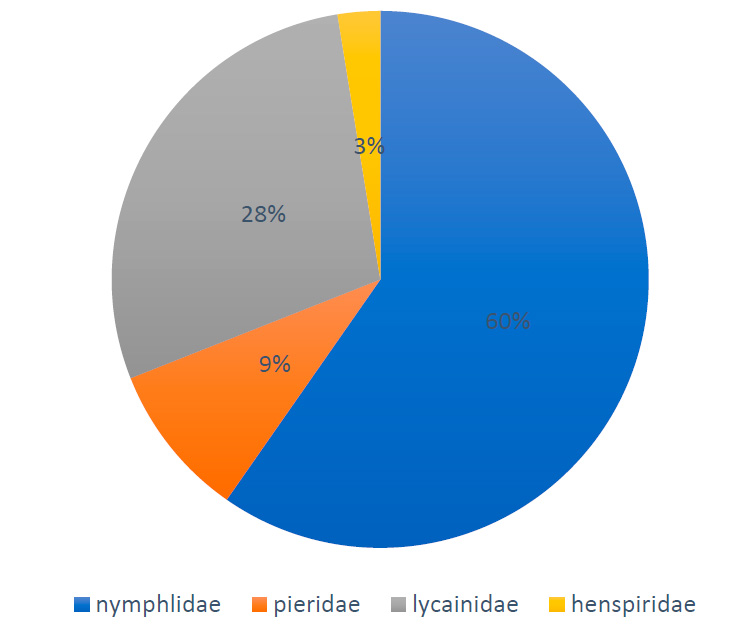
Figure 1. Relative abundance of butterflies’ families associated with the Brassicaceae crops in Basra during the growing season of 2020-2021.
Butterflies Biodiversity (Ecological indices)
Spatial Diversity
The results of Table (6) indicated that the ecological indices had a slight variation between the study stations; the highest values of Diversity and richness were 1.79 and 1.19 at Karmat Ali, compared to the values of 1.65 and 0.96 in the Al-Zubair region. The highest values of dominance and Evenness were 0.40 and 0.75 in the Al-Zubair region, and the lowest values of 0.38 and 0.66, respectively, were in Karmat Ali.
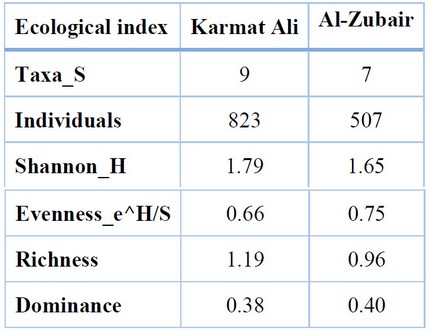
Table 6. Butterflies Biodiversity in Karmat Ali and Al-Zubair regions, 2020-2021.
Temporal Diversity
The results of the temporal Diversity of butterfly species in the Karmat Ali region (Table 7) indicated that the butterflies’ highest value of Diversity was 1.80 in November; however, the lowest value was 1.45 in February. The highest value of richness (1.47) was recorded in March, and the lowest (1.19) was in November. The results revealed butterflies’ lowest degree of dominance (0.29) and highest evenness value (0.86) in February. In contrast, the highest value of dominance (0.53) and lowest value of Evenness of species (0.54) was found in November.
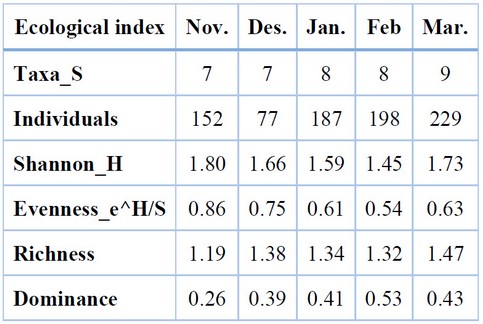
Table 7. Butterflies biodiversity during the growing season at Karmat Ali region, 2020-2021.
At the Al-Zubair region (Table 8), the temporal Diversity of butterfly species differed from that of the Karmat Ali site; the highest and lowest values of Diversity were 1.80 and 1.21 in January and February, respectively. The lowest value of richness was recorded in January (1.32), while the lowest was found in November (0.52). In December, the highest degree of dominance (0.46) and lowest Evenness (0.72) of butterflies were recorded. However, the lowest value of dominance (0.28) and highest Evenness (0.96) was found in November and January, respectively.
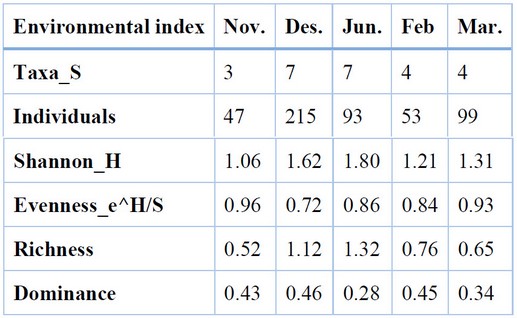
Table 8. Butterflies Biodiversity during the growing season at Al-Zubair region, 2020-2021.
DISCUSSION
The community composition of butterflies associated with Brassicaceae plants was very similar depending on the butterfly species at the Karmat Ali and Al-Zubair sites; nine species of butterflies were recorded belonging to four families of the order Lepidoptera; the results of the study indicated that the family Nymphalidae was the highest relative abundant, followed by Lycainidae and Pieridae; while Hesperiidae was lower abundant (Figure 1); Nymphalidae is represented by two important species, V. carudi and Danaus chrysippus, which prefer open field cultivations compared to greenhouses 43; in this study, V. carudi which is one of the relative generalist species 43 had the highest butterfly population and was the most abundant throughout the sites, while P. rapae was less abundant and had low density during the growing season. The current study’s findings indicated that a small number of butterfly species occurred in Brassicas fields in both sites of the sedimentary and desert habitats. Several investigations indicated that agroecosystem habitats usually supported poor communities of butterfly species 44, 45, 46 explained that the agroecosystem usually supported very few butterfly species (generally 5–50), which is characterized by a high population of butterflies. However, another study about the butterflies in Dohuk Province, northern Iraq, showed the presence of 49 species belonging to seven families: Papilionidae, Pieridae, Libytheidae, Nymphalidae, Satyridae, Lycaenidae and Hesperiidae 32.
The seasonality results indicated that the highest rate of butterflies was recorded in the Karmat Ali area in March, and the lowest number was found in December. In contrast to the Al-Zubair site, the highest and lowest populations of butterflies were observed in December, and the lowest populations were recorded in February (Table 3-5). Depending on field observation, the most abundant butterfly, V. carudi(Nymphlidae), a generalist species, was regularly recorded collecting floral resources on several weeds in the blooming stage; they were observed making extended visits to flowers of Malow Malva parviflora in December in Al-Zubair. The results of this study confirmed that the 1st site (Karmat Ali), cultivated with different plantations and located at the river edge, had high Diversity and population densities. In contrast, the 2nd site (Al-Zubair), which had an enormous monoculture plantation, stressed fewer species of butterfly community 47, 48. However, the seasonal variation of butterfly species populations at these two different sites could be attributed to the cyclic changes in climate elements of temperature and precipitation monthly 49-51. The butterflies’ seasonal activity usually coincides with the peak of the flowering stage of host plants52-54.
Overall, butterflies’ Diversity and richness varied between the study sites; the Karmat Ali site hosted a higher diversity and richness of butterfly species than the Al-Zubair site. The differences in butterflies’ richness and Diversity were attributed to the differences in region characteristics (agricultural landscape, plantations, and river vicinity of Brassica plants), in addition to the influence of environmental factors 55.
CONCLUSIONS
The lack of large areas of natural habitats in Basra province and the incidence of rare and uncommon butterfly species pushes it urgent that efforts should be focused on conserving biodiversity through the maintenance and improvement of natural and agricultural habitats; it was noticed that P. rapae, which was abundant years ago in Basra, is the least abundant now. The current study offers specific encouragement that the Brassicaceae plants support the butterfly’s community even with a small number of butterfly species; nine species belong to Nymphlidae, Lycainidae, Pieridae, and Hesperiidae associated with the above plant family. Due to the findings, the butterfly species have specific habitat requirements; the species diversity of butterflies in the agroforestry systems of Karamat Ali (sedimentary habitats) is much higher than that of the Al-Zubair region (desert habitats); the study of butterflies abundance and biodiversity indices indicated that environmental factors and the polyculture plantations support the butterfly’s population in the natural and agricultural habitats. Depending on the present study, the use of butterfly species indicates the agricultural landscape’s biodiversity.
Author Contributions: Conceptualization, F. N. J. and A.A.; H. H. A methodology, F. N. J. and A.A.; H. H. A; software, A.M.M.; validation, F. N. J. and A.A.; formal analysis, A.M.M. and A.A.; investigation, A.M.M. and A.A.; resources, A.M.M. and A.A.; data curation, F. N. J. and A.A.; writing—original draft preparation, F. N. J. and A.A.; writing—review and editing, A.A.; visualization, A.A.; supervision, A.A.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: The data supporting this study’s findings are available from the corresponding author, Aqeel Alyousuf, upon reasonable request.
Acknowledgments: The authors thank the growers who let us conduct this study in their orchards.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Kumar S, Simonson SE, Stohlgren TJ. Effects of spatial heterogeneity on butterfly species richness in Rocky Mountain National Park, CO, U.S.A. Biodivers. Conserv. 2009;18(3):739-63. https://doi.org/10.1007/s10531-008-9536-8
2. Kremen C. Assessing species assemblage indicator properties for monitoring natural areas. Ecol Appl .1992;2(2):203-17. https://doi.org/10.2307/1941776
3. Kremen C. Biological inventory using target taxa: a case study of the butterflies of Madagascar. Ecol Appl. 1994;4(3):407-22. https://doi.org/10.2307/1941946
4. Van Swaay CA, Dennis EB, Schmucki R, Sevilleja C, Balalaikins M, Botham M, et al. The E.U. Butterfly Indicator for Grassland species: 1990-2017. Technical report. 2019. https://doi.org/10.13140/RG.2.2.34330.98240
5. Schmidt N, Roland J. Moth diversity in a fragmented habitat: importance of functional groups and landscape scale in the boreal forest. Annals of the Entomological Society of America. 2006;99(6):1110-20.https://doi.org/10.1603/0013-8746(2006)99[1110:MDIAFH]2.0.CO;2
6. Sharma M, Sharma N. Suitability of butterflies as indicators of ecosystem condition: a comparison of butterfly diversity across four habitats in Gir Wildlife Sanctuary. Int. J. Adv. Res. Biol. Sci.2017;4(3):1-12.
7. Yu-Feng M, Khan Z, Perveen F, Rafi MA, Shah SW, Xiao-Hong S, et al. Biodiversity of butterflies in Tangi Charsadda, Khyber Pakhtunkhwa, Pakistan. Pak. J. Zool .2020;52(3):835. https://dx.doi.org/10.17582/journal.pjz/20190326090338
8. Brito MM, Ribeiro DB, Raniero M, Hasui E, Ramos FN, Arab A. Functional composition and phenology of fruit-feeding butterflies in a fragmented landscape: Variation of seasonality between habitat specialists. J. Insect Conserv. 2014;18(4):547-60.
9. Sharma S, Dalip K, Mansotra JP. Role of butterflies in shaping an ecosystem: why to protect them. Biodivers. Conserv. 2020;39:44.
10. Sagi N, Hawlena D. Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems. Insects. 2021;12(8):726. https://doi.org/10.3390/insects12080726
11. Kagata H, Ohgushi T. Carbon to nitrogen excretion ratio in lepidopteran larvae: relative importance of ecological stoichiometry and metabolic scaling. Oikos. 2012;121(11):1869-77. https://doi.org/10.2307/41686652
12. Hanspach J, Loos J, Dorresteijn I, von Wehrden H, Moga CI, David A. Functional diversity and trait composition of butterfly and bird communities in farmlands of Central Romania. Ecosyst. Health Sustain 2015;1(10):1-8. https://doi.org/10.1890/EHS15-0027.1
13. van Nouhuys S, Shaw M, Stefanescu C. Parasitoids of European butterflies. Ecology of butterflies of Europe: Cambridge University Press; 2009. p. 130-56.
14. Toro-Delgado E, Hernández-Roldán J, Dincă V, Vicente JC, Shaw MR, Quicke DL, et al. Butterfly–parasitoid–hostplant interactions in Western Palaearctic Hesperiidae: a D.N.A. barcoding reference library. Zoological Journal of the Linnean Society. 2022. https://doi.org/10.1093/zoolinnean/zlac052
15. Warren MS, Maes D, van Swaay CAM, Goffart P, Van Dyck H, Bourn NAD, et al. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proceedings of the National Academy of Sciences. 2021;118(2):e2002551117. https://doi.org/10.1073/pnas.2002551117
16. Forister M, Halsch C, Nice C, Fordyce J, Dilts T, Oliver J, et al. Fewer butterflies seen by community scientists across the warming and drying landscapes of the American West. Science. 2021;371(6533):1042-5. https://doi.org/10.1126/science.abe5585
17. Robinet C, Roques A. Direct impacts of recent climate warming on insect populations. Integrative zoology. 2010;5(2):132-42. https://doi.org 10.1111/j.1749-4877.2010.00196.x
18. University of Arizona. Dramatic decline in western butterfly populations linked to fall warming. ScienceDaily Retrieved September 7, 2022 from wwwsciencedailycom/releases/2021/03/210304145405htm. 2021. www.sciencedaily.com/releases/2021/03/210304145405.htm.
19. Stefanescu C, Torre I, Jubany J, Páramo F. Recent trends in butterfly populations from north-east Spain and Andorra in the light of habitat and climate change. Journal of Insect Conservation. 2011;15(1):83-93. https://doi.org/10.1007/s10841-010-9325-z
20. Fox R, Brereton T, Asher J, August T, Botham M, Bourn N, et al. The State of the U.K.’s Butterflies 2015. 2015.
21. Gonzalez D, Pinto L, Sousa D, Oliveira I, Oliveira PS. Butterfly species richness and Diversity on tourism trails of Northeast Portugal. Journal of Entomological Science. 2017;52(3):248-60.
22. Thogmartin WE, Wiederholt R, Oberhauser K, Drum RG, Diffendorfer JE, Altizer S, et al. Monarch butterfly population decline in North America: identifying the threatening processes. Royal Society open science. 2017;4(9):170760. https://doi.org 10.1098/rsos.170760
23. Munyuli MT. Pollinator biodiversity in Uganda and in Sub-Sahara Africa: Landscape and habitat management strategies for its conservation. International Journal of Biodiversity and Conservation. 2011;3(11):551-609.
24. Ellis S. U.K. Conservation Strategy 2025 Appendix 5 U.K. Landscape Conservation Progress. 2018. . https://doi.org 10.1017/S1742758409990233
25. Kuefler D, Haddad NM, Hall S, Hudgens B, Bartel B, Hoffman E. Distribution, population structure and habitat use of the endangered Saint Francis Satyr butterfly, Neonympha mitchellii francisci. The American Midland Naturalist. 2008;159(2):298-320. https://doi.org 10.1674/0003-0031(2008)159[298:DPSAHU]2.0.CO;2
26. Jansen SH, Holmgren M, van Langevelde F, Wynhoff I. Resource use of specialist butterflies in agricultural landscapes: conservation lessons from the butterfly Phengaris (Maculinea) nausithous. Journal of Insect Conservation. 2012;16(6):921-30. https://doi.org 10.1007/s10841-012-9479-y
27. Brückmann SV, Krauss J, Steffan-Dewenter I. Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. Journal of Applied Ecology. 2010;47(4):799-809. https://doi.org/10.1111/j.1365-2664.2010.01828.x
28. Vargas H, Benítez H. Egg Phenology of a Host-Specialist Butterfly in the Western Slopes of the Northern Chilean Andes. Neotropical Entomology. 2013.
29. Kemal M, Koçak A. A synonymous and distributional list of the Lepidoptera of Iraq based on the infosystems of the Cesa. Priamus. 2018;17(2114):208.
30. Augul RS. Insect pollinators in different regions of Iraq. Journal of Entomology and Zoology Studies. 2016;4(2):391-402.
31. Khudhur FA. Faunistic study of butterflies (Lepidoptera, Papilionoidea) of Sulaymaniyah Province, Kurdistan-Iraq. Biodiversity Data Journal. 2022;10. https://doi.org 10.3897/BDJ.10.e82612
32. Said NT, Çiftçi MC, Seven E. A preliminary list of butterflies (lepidoptera, rhopalocera) from Duhok (Northern Iraq). Transactions of the American Entomological Society. 2018;144(3):637-44. https://doi.org/10.3157/061.144.0313
33. Katumo DM, Liang H, Ochola AC, Lv M, Wang Q-F, Yang C-F. Pollinator diversity benefits natural and agricultural ecosystems, environmental health, and human welfare. Plant Diversity. 2022. https://doi.org 10.1016/j.pld.2022.01.005
34. Stanley DA, Gunning D, Stout JC. Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: ecological and economic incentives for pollinator conservation. Journal of Insect Conservation. 2013;17(6):1181-9. https://doi.org 10.1007/s10841-013-9599-z
35. A. Al-ani, H., S. Asker, A., S. Hseen, N. The Effect Of Using Anabasis Articulate Extract And Melatonin Hormone Locally In Vaginal Sponges On The Reproductive Performance Of Local Iraqi Ewes. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 214-223. doi: 10.32649/ajas.2023.179739.
36. Abbas M. Distribution and taxonomy of butterflies of district Skardu [NWFP] Pakistan. 2000.
37. Wiltshire EP. Lepidoptera of Iraq.The Ministry of Agriculture Government of Iraq, Nicholas Kaye, Ltd, London. 1957;162.
38. Ali, H.H., AL-Rawi, K., Khalaf, Y., Alaaraji, S., Aldahham, B., Awad, M., Al-ani, O., Al-ani, F., Ali, A.T. Serum Caveolin-1 Level is Inversely Associated with Serum Vaspin, Visfatin, and HbA1c in Newly Diagnosed Men with Type-2 Diabetes (2022) Reports of Biochemistry and Molecular Biology, 11 (2), pp. 299-309.
39. Shannon E, Wiener W. The mathematical theory of communication university of Illinois press Urbana Illinois, pp: 117. 1963.
40. Margalef R. Diversity and stability: a practical proposal and a model of interdependence. Bookhaven Symp Biol. 1969;22:25-37.
41. Pielou EC. Mathematical ecology. John wiely, New York 385pp. 1977.
42. Omar Khaled Attallah, Thafer Thabit Mohammed and Nasr Nuri Al-Anbari. Effect of Adding Grape Pomace and Resveratrol on Some Physiological Traits and Gene Expression to Prevent Hemorrhagic Fatty Liver Syndrome in Laying Hens . I.O.P. Conference Series: Earth and Environmental Science.2022, 1060 (1), 012076. doi:10.1088/1755-1315/1060/1/0120
43. Cuadrado M. Assessing year-round phenology and reproduction of the migratory painted lady butterfly, Vanessa Cardui (Lepidoptera: Nymphalidae), in a Mediterranean area in southern Spain. European Journal of Entomology. 2021;118. https://doi.org/ 10.14411/eje.2021.029
44. Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
45. van Swaay CA, Nowicki P, Settele J, Van Strien AJ. Butterfly monitoring in Europe: methods, applications and perspectives. Biodiversity and Conservation. 2008;17(14):3455-69. https://doi.org/ 10.1007/s10531-008-9491-4
46. Elbers JP, Bossart J. Occurrences of forest butterflies in the farm bush savannah outside a forest reserve in Ghana, West Africa. International Journal of Tropical Insect Science. 2009;29(3):141-50. https://doi.org/ 10.1017/S1742758409990233
47. Panjaitan R, Drescher J, Buchori D, Peggie D, Harahap IS, Scheu S, et al. Diversity of butterflies (Lepidoptera) across rainforest transformation systems in Jambi, Sumatra, Indonesia. Biodiversitas Journal of Biological Diversity. 2020;21(11). https://doi.org/ 10.13057/biodiv/d211117
48. Ghanim, I. .; Ebrahim, S. E. . Preparing Of Bio-Cement Mortar By Using Bacillus Licheniformis Bacterial Cells. JLSAR 2022, 3, 23-29
49. Checa MF, Levy E, Rodriguez J, Willmott K. Rainfall as a significant contributing factor to butterfly seasonality along a climatic gradient in the neotropics. BioRxiv. 2019:630947. https://doi.org/10.1101/630947
50. Halali S, Halali D, Barlow HS, Molleman F, Kodandaramaiah U, Brakefield PM, et al. Predictability of temporal variation in climate and the evolution of seasonal polyphenism in tropical butterfly communities. 2021. https://doi.org/10.1111/jeb.13895
51. Grøtan V, Lande R, Engen S, Saether B-E, Devries P. Seasonal cycles of species diversity and similarity in a tropical butterfly community. The Journal of Animal Ecology. 2012;81:714-23. 10.1111/j.1365-2656.2011.0195.x
52. Toftegaard T, Posledovich D, Navarro Cano J, Wiklund C, Gotthard K, Ehrlén J. Butterfly-host plant synchrony determines patterns of host use across years and regions. Oikos. 2018;128. https://doi.org/10.1111/oik.05720
53. Wiklund C, Åhrberg C. Host Plants, Nectar Source Plants, and Habitat Selection of Males and Females of Anthocharis cardamines (Lepidoptera). Oikos. 1978;31(2):169-83.
54. Ghosh S, Saha S. Seasonal diversity of butterflies with reference to habitat heterogeneity, larval host plants and nectar plants at Taki, North 24 Parganas, West Bengal, India. World Scientific News. 2016(50):197-238.
55. Posledovich D, Toftegaard T, Wiklund C, Ehrlén J, Gotthard K. Phenological synchrony between a butterfly and its host plants: Experimental test of effects of spring temperature. Journal of Animal Ecology. 2017; 87. https://doi.org/10.1111/1365-2656.12770
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Jaber, F. N., Alyousuf, A. and Al-Saffar, H. Diversity of Butterflies associated with Brassicaceae crops in Basra, Iraq. Revis Bionatura 2023;8 (4) 62. http://dx.doi.org/10.21931/RB/2023.08.04.62



















