Vol 8 No 4 2023 – 45
Wurood Hantoosh Neamah 1*, Fatimah Ali Hasan2
1 University of Basrah/ Basrah/ Iraq;
2 University of Basrah/ Basrah/ Iraq; fatimah.hasan@uobasrah.edu.iq;
* Correspondence: wurood.neamah@uobasrah.edu.iq; Tel.: (+9647819865272)
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.50
ABSTRACT
Among medicinal plants humans use, fennel (Foeniculum vulgare) is essential due to its flavor and health benefits. A clean environment requires sustainable methods to reduce soil, water, and air pollution. Camel thorn (Alhagi maurorum) is a desert plant distributed widely in Iraq. This experiment investigated the effect of A. maurorumextract spraying on vegetative, floral, and seed numbers of F. vulgare. 0, 1.5 and 3 g L-1 concentrations of shoot root extracts of A. maurorumwere utilized as a foliar application on F. vulgare.A significant increase in studied characteristics of F. vulgare was obtained under extract treatments. Spray with 3 and 1.5 g L-1 of shoot root extracts obtained the highest number of seeds per plant. The results of the present study exhibited a potential to use A. maurorum as a natural fertilizer.
Keywords: Foeniculum vulgare; Alhagi maurorum; foliar application; natural fertilizer; flavonoids; essential oil.
INTRODUCTION
Foeniculum vulgare Mill or fennel is a plant with dietary, culinary, and medicinal uses that belongs to the Umbelliferae (Apiaceae) family; the origin country of the plant is the Mediterranean area, but it is now cultivated almost in every country. It is known to be ancient and used by humans for its flavor and health benefits. 1. F. vulgare, a plant, is rich in nutrients, including fibers, carbohydrates, sugar, vitamins, protein, energy, and minerals distributed in whole plant parts and related to human daily needs; pulpy shoots are still edible vegetables in southern Italy 2. Fennel seeds are used in preparing fish and meat dishes due to their flavor; bulb roots are used as salad, snacks, stewed, boiled, and grilled, and refreshing tea is also prepared from seeds and leaves of plant 3. Pharmacological activities of F. vulgare are related to phytoconstituents content that include numerous important compounds of plant secondary metabolism such as flavonoids, polyphenols, fatty acids, and volatile compounds 4. Volatile compounds are present mainly in essential oils that give the plant an odor and desired flavor, making the food and food products an excellent test. The number and type of volatile compounds are variables according to solvent, plant part, and extraction technique 5-7. However, anethole is considered the primary volatile compound in the essential oil of fennel plant 8.
F. vulgare is officially noted in Ayurvedic pharmacopeia and the Canon of Medicine as an essential component of the herbal mixture used to treat numerous illnesses and disorders. The essential oil and extracts of F. vulgare seeds exhibited a broad spectrum of bacterial and viral inhibition9-12. Antiinflammatory and antiallergic properties of methanolic extract of F. vulgare fruit are investigated and exhibited a significant reduction effect in inflammation and hypersensitivity in vivo13, 14. Wild fennel exhibits a higher antiaging activity than medicine and edible fennel by scavenging free radicals caused by oxidative stress 15. Additionally, F. vulgare extracts and essential oil are reported to have other pharmacological activities, including estrogenic and galactogenic activities 16, oculohypotensive activity 17, antimutagenic effect 18, gastrointestinal protective 19, hepatoprotective role 20, and hypoglycemic activity 21.
Alhagi maurorumor camel thorn or Aqool (plant’s common name in Iraq)is a desert plant belonging to the Fabaceae (Leguminosae) family. It grows in a wide area, including many Asian countries, Africa, North America and Europe 22. Because of its deep root system that reaches six or seven feet into the ground, the plant can grow in dry areas with high salinity and alkalinity 23. In some countries, plant growth is controlled as a weed by several methods. However, A. maurorum is rich in critical phytochemical compounds, secondary metabolites that increase under stress, such as flavonoids, fatty acids, coumarins, carbohydrates, tannins, unsaturated sterols, glycosides, sterols, steroids, resins, minerals, vitamins, alkaloids and triterpenes 22-24. Treatment of winter and summer crops with aqueous extract of A. maurorum caused an increase in total chlorophyll, carotenoids, glutathione, and protein content25. Also, a recent study found that treating sativum L. with an aqueous extract of Alhagi maurorum led to an increase in soluble sugars, soluble protein, proline, and flavonoids 26. The present study was interested in examining the foliar application of shoot and root extract of A. maurorum on F. vulgare growth and investigating the possibility of using the extracts as alternative growth stimulators to chemical synthetic fertilizers due to cheap cost and sustainability features.
MATERIALS AND METHODS
Fennel seeds were obtained from the local market in Basrah and planted in trays with peat moss substrate in December in the greenhouse / Agriculture College / University of Basrah. After the seedlings reached 5 cm, they were transplanted into pots with a 35 cm diameter and a 3:1 soil-to-peat moss ratio.
Alhagi maurorum extraction
Camelthorn plants were collected from the University of Basrah location; plants were uprooted, washed well, and dried at room temperature. Plants were separated into shoot and root parts, ground until soft powder, weighed 1.5 or 3 g from both parts and soaked with 1 L of distilled water overnight. The next day, extracts are filtrated by filter paper to prepare for the foliar spray21.
Foliar application
When fennel plants reached 15 cm, they were sprayed with 1.5 or 3 gL-1 of shoot extract of A. maurorum—the next day, 1.5 or 3 g L-1 of root extracts were applied to plants. The foliar application was repeated 3 times on plants, and the period between each spray was one month.
Growth rates measurement
Vegetative characteristics
Plant height (cm)
The height of each plant was measured after the flowering stage by tape measuring from the surface of the potting substrate to the top of the plant and recording.
Branches and leaves number plant-1.
The total of branches and leaves is counted and recorded for each plant.
Fresh weight (g)
A four-level sensitive scale recorded the fresh weight of each plant.
Dry weight (g)
Plants were dried at room temperature until dry mass stability and a four-level sensitive scale recorded weight.
Floral and seed characteristics
Inflorescence plant-1
The number of inflorescences for each plant was counted and recorded after the complete formation of inflorescences.
Flowers plant-1
The number of flowers in inflorescences for each plant was recorded after the flowering stage.
Seeds number plant-1
The total number of seeds is counted after they dry and recorded for each plant.
Experiment design and statistical analysis
The experiment includes 9 factorial treatments, the interaction between two factors. First is the spray with shoot extract of A. maurorum extract at (0, 1.5, 3) gL-1 concentration and the second is the spray with root extract of A. maurorum at (0, 1.5, 3) gL-1 concentrations. The experiment was designed according to the randomized complete block design (R.C.B.D.) for a factorial experiment. The experiment was repeated twice with three plants for each treatment. Thus, the total number of factorial experiment plants was 54. The graphprism program was used for results analysis and probability level. 0.05 was used to compare averages by one-way analysis of variance ANOVA.
RESULTS
Vegetative characteristics
Plant height
Figure (1) illustrates the effect of study factors and their interaction on the F. vulgare height. Both study factors and their interaction significantly affect plant height compared to control (V0R0). Spray with 3 gL-1 of A. maurorum root extract (V0R2) obtained the highest plant height, 63 cm, compared to V0R0. Also, the spray with 3 gL-1 of A. maurorumshoot extract (V2R0) caused an increase in plant height of 60 cm; the interactions between the two study factors led to a significant increase in plant height compared to the control, 45 cm (Figure 1).
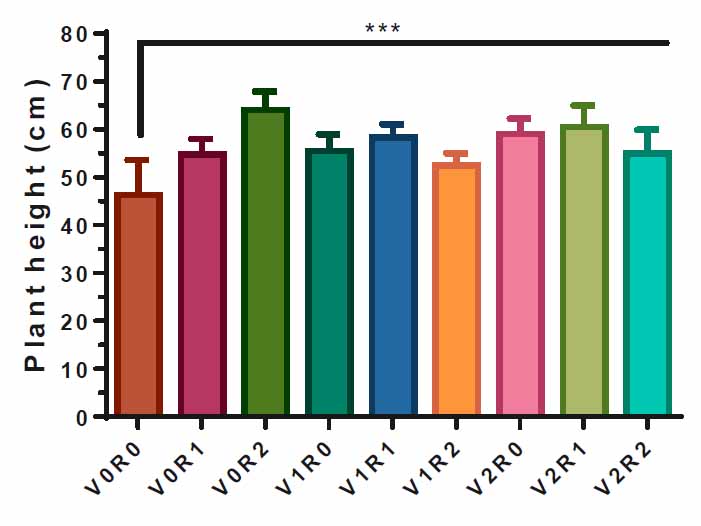
Figure 1. Study factors’ effect and interaction on F. vulgare height.
The effect of spraying with study factors and their interaction on the height of F. vulgarewas recorded after the flowering stage. V0R0 is 0 gL-1 of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 gL-1 of A. maurorum root extract, V0R2 is 3 gL-1 of A. maurorum root extract, V1R0 is 1.5 gL-1 of A. maurorumshoot extract, V1R1 is the interaction between 1.5 gL-1 of shoot extract and 1.5 gL-1 of root extract, V1R2 is the interaction between 1.5 gL-1of shoot extract and 3 gL-1 of root extract, V2R0 is 3 gL-1of shoot extract of A. maurorum, V2R1 is the interaction between 3 gL-1 of shoot extract and 1.5 gL-1 of root extract, V2R2 is the interaction between 3 gL-1 of both shoot& root extracts. A multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned as follows: ***p < 0.001.
Branches and leaves number.
The study factors and their interaction negatively affected the number of branches and leaves per plant (figure 2).
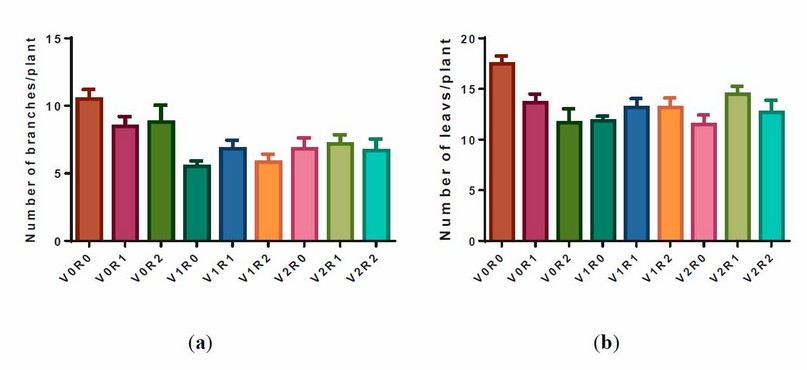
Figure 2. Effect of study factors and their interaction on the number of branches and leaves of F. vulgare.
(a) A representative diagram of the effect of study factors on branch number was recorded after the flowering stage. (b) Representative diagram of the effect of study factors on leaves number recorded after flowering stage. V0R0 is 0 g L-1of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1 of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorum shoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract and 1.5 g L-1 of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract and 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract & 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract and root extract.
Fresh weights
The plants sprayed with 3 gL-1 of A. maurorum shoot extract V2R0 recorded the highest fresh weight, 33 g, compared to control V0R0, 19g. Spraying the plants with 1.5 gL-1 of A. maurorum root and shoot extracts caused a significant increase in the fresh weight of F. vulgare, 25 g and 27g, respectively. The interaction of study factors did not significantly affect the fresh weight trait (Figure 3).
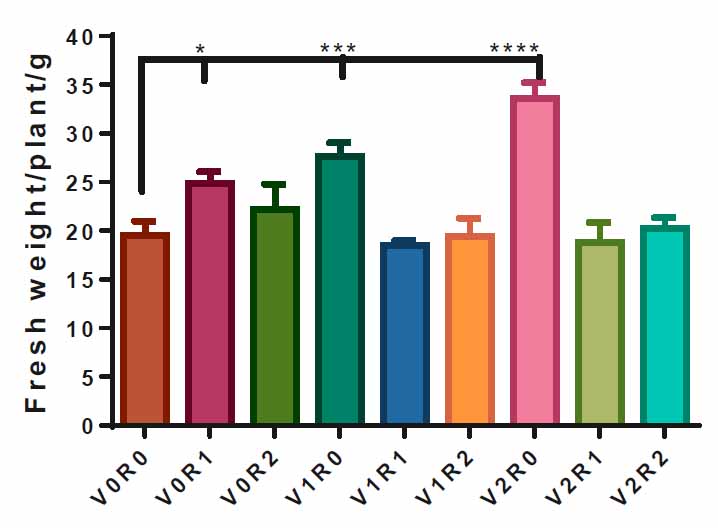
Figure 3. Effect of study factors and their interaction on fresh weight of F. vulgare.
Representative, the effect of spraying with study factors and their interaction on F., vulgare fresh weight. V0R0 is 0 g L-1 of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1 of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorumshoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract & 1.5 g L-1 of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract and 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract and 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract &root extract. Multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned: *p < 0.05, ***p < 0.001, ****p < 0.0001.
Dry weight
All F. vulgare sprayed with 1.5 gL-1 and 3 gL-1 of roots and shoot extracts of A. maurorum show a significant increase in dry weight compared to the control, 3.1 g. However, the spraying with 1.5 gL-1 of root extract and 3 gL-1 of shoot extract achieved the highest dry weight of the plant, 11 g, compared to the control (Figure 4).
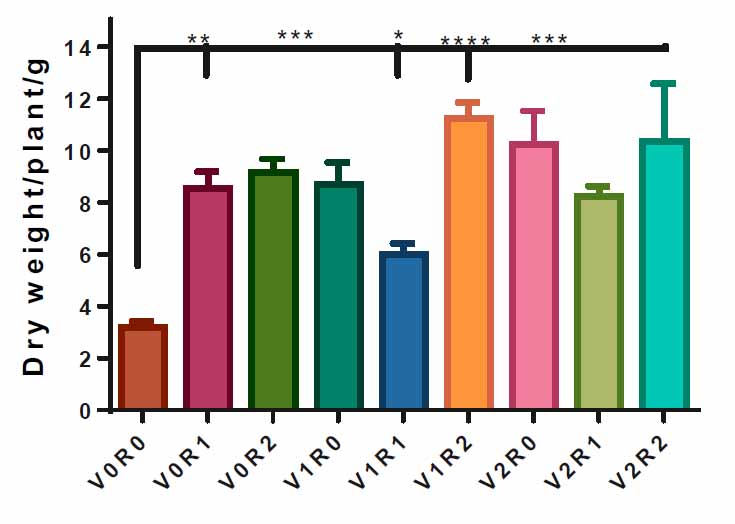
Figure 4. Effect of study factors and their interaction on the dry weight of F. vulgare.
The effect of spraying with study factors and their interaction on F. vulgarity weight was recorded after total plant drying. V0R0 is 0 g L-1 of shoot extract and root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1 of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorum shoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract and 1.5 g L-1of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract& 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract & 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract & root extract. A multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned as following: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Floral and seed characteristics
Number of inflorescences
Spraying with 1.5 gL-1 of shoot & root extract V1R1 and 3 gL-1 of both extracts V2R2, as well as a spray with 3 gL-1 of shoot extract led to the highest number of inflorescences in treated plants, 13 inflorescenceplant-1 compared to control V0R0, 10 inflorescenceplant-1. However, study factors and other interactions have a positive effect on the number of inflorescences per plant (Figure 5)
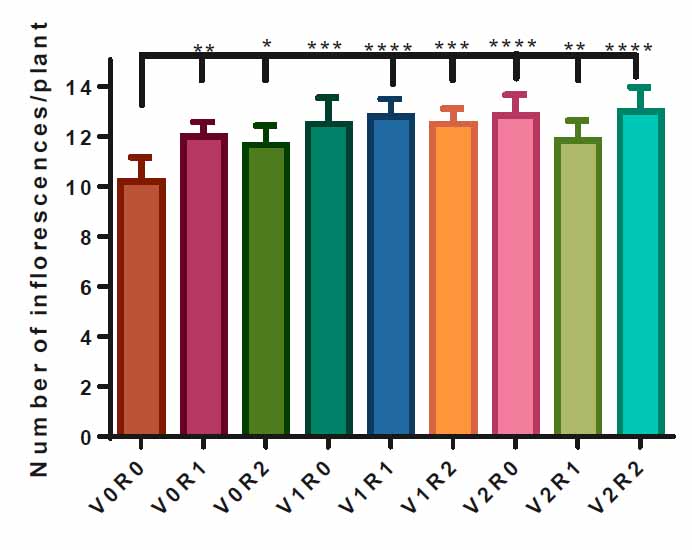
Figure 5. Effect of study factors and their interaction on inflorescences number of F. vulgare.
The effect of spraying with study factors and their interaction on the number of inflorescences of F. vulgarewas recorded after the complete formation of inflorescences. V0R0 is 0 g L-1 of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorum shoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract & 1.5 g L-1 of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract and 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract & 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract and root extract. A multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned as following: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Number of flowers
F. vulgare that was sprayed with 1.5 g L-1 of root extract&1.5 gL-1 or 3 g L-1 of shoot extract V1R1& V2R1 of A. maurorum exceeded of flowers number per plant, 110 and 117 flower plant -1 respectively compared to control V0R0, 73 flower plant-1. Also, spraying with 3 g L-1 of shoot extract significantly increased 100 flower plant-1 compared to the control group (Figure 6).
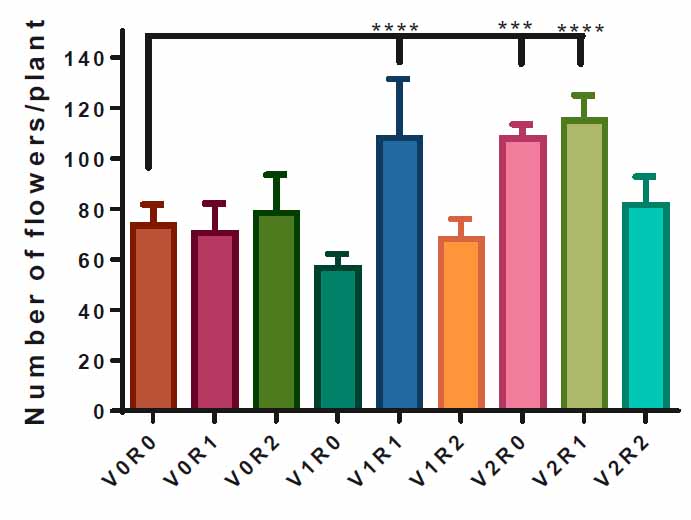
Figure 6. Effect of study factors and their interaction on flower number of F. vulgare.
The effect of spraying with study factors and their interaction on several F. vulgare flowers was recorded after the flowering stage. V0R0 is 0 g L-1 of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1 of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorum shoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract & 1.5 g L-1 of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract and 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract & 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract & root extract. A multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned as follows: ***p < 0.001, ****p < 0.0001.
Number of seeds
The study factors and their interaction positively affected the number of seeds per plant. Figure 7 elucidates that individual and interaction treatments significantly increased seed number except for the V1R0 treatment, which does not affect the seed number of treated plants compared to the control treatment. Plants sprayed with 1.5 gL-1 of root extract and 3 gL-1 of shoot extract of A. maurorum recorded the highest number of seeds per plant, 105 seedplant-1, compared to the control treatment V0R0, 44 seedplant-1 (Figure 7).
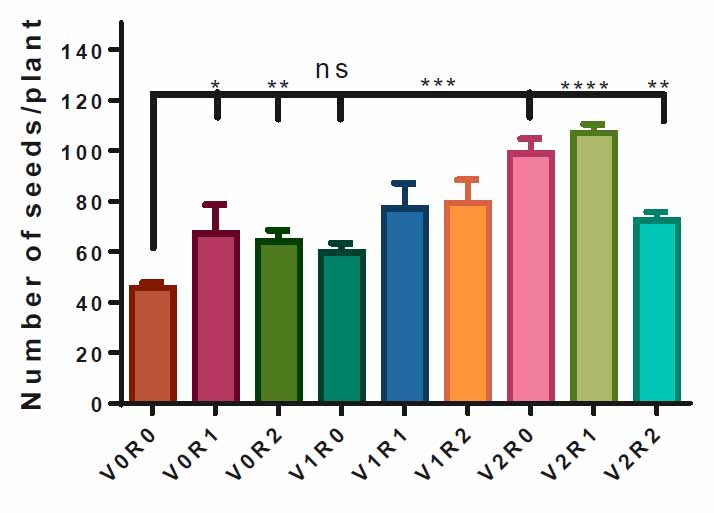
Figure 7. Effect of study factors and their interaction on seeds number of F. vulgare.
The effect of spraying with study factors and their interaction on the number of seeds of F. vulgare was recorded after the seeds were dried. V0R0 is 0 g L-1 of shoot extract root extract of A. maurorum (control treatment), V0R1 is 1.5 g L-1 of A. maurorum root extract, V0R2 is 3 g L-1 of A. maurorum root extract, V1R0 is 1.5 g L-1 of A. maurorum shoot extract, V1R1 is the interaction between 1.5 g L-1 of shoot extract & 1.5 g L-1 of root extract, V1R2 is the interaction between 1.5 g L-1 of shoot extract & 3 g L-1 of root extract, V2R0 is 3 g L-1 of shoot extract of A. maurorum, V2R1 is the interaction between 3 g L-1 of shoot extract and 1.5 g L-1 of root extract, V2R2 is the interaction between 3 g L-1 of both shoot extract & root extract. A multiple ANOVA was performed using ordinary 0ne-way ANOVA multiple comparisons. Significance was assigned as following: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
DISCUSSION
The obtained result exhibits an increase in the plant’s height with A. maurorum extract spray (Figure 1). ). Increase the height of fennel plants when sprayed with shoot and roots of A. maurorum or camelthorn extracts due to the rich content of phenolic compounds such as 1,3-Butanadiol,1,3-butylene glycol, 4-Fluoroveratrole, fluorobenzene,3,4-methoxy and 2,3-Dimethylpenzene 27 Phenolic compounds are responsible for the growth of the plant by helping in cell wall formation, these pools of wall-bound phenolic compound act as a reservoir of phenylpropanoid units for lignin biosynthesis or they act as representative to beginnings of lignification itself 28. However, spraying. Vulgare with shoot and root extracts of A. maurorum caused a decrease in the number of branches and leaves of plants compared to the control (Figure 2). The diminishing number of branches and leaves may be due to containing A. maurorumextracts on Coumarin that have a regulatory effect on leaves and branches growth 29 or due to the direction of plant growth to elongation at the expense of shoots growth. Figure 3 shows the fresh weight of fennel plants after the treatment with A. maurorum extract. The spraying with 1.5 gL-1 of shoot extract only and roots extract only, as well as with 3 gL-1 of shoot extract of A. maurorum caused a significant increase in the fresh weight of fennel plants (27, 25, 33) g respectively compared to control 20 g without any effect for study factors interaction (Figure 3). At the same time, the spraying with all concentrations of shoot and root extracts of A. maurorum led to a significant increase in the dry weight of F. vulgare compared to the control (Figure 4). The increase in the weight of plants, particularly dry weight, maybe because of the high content of carbohydrates (56.52%) in A. maurorum extract, in addition to trace elements present, including Ca, Mg, K, Na, Fe, Cu, Zn, Cr, Cd, Pb, and Ni 22, foliar spray with carbohydrate and elements source (A. maurorum extract) enhanced plants growth that positively reflected on plants weigh 30, 31.
Our result showed an increase in inflorescence numbers after foliar spraying with shoot root extracts of A. maurorum compared to the control group (Figure 5). At the same time, the number of flowers per plant was affected by only spraying with 3 gL-1 of shoot extract. The interaction between 1.5 gL-1 of both shoot and root extracts and interaction between 3gL-1 and 1.5 gL-1 of shoot extract and root extract of A. maurorum respectively (Figure 6). A. maurorum is a legumes plant that can form a symbiotic relationship with nitrogen-fixing soil bacteria called rhizobia. A. maurorum has a high content of flavonoids including chrysoeriol-7-O.-xylosoid, kaempferol-3-galactorhamnoside isorhamnetin, kaempferol, isorhamnetin 3-O.-β-D-apio-furanosyl (1-2) β-D-galactopyranoside 22. Flavonoids are secondary metabolites and have a defense role in the plant. Flavonoids that are secreted in the roots play an additional function in initiating symbiotic development in most legumes 32 by acting as mediators in NodD protein synthesis and stimulators of NodD linkage to nod gene promoters in Sinorhizobium meliloti leading to enhancement of nitrogen-fixing 33. Nitrogen is essential in plant growth at all stages, as it is known that the flowering stage requires a low level of nitrogen and a high level of phosphor. However, several kinds of literature indicated that sufficient or high level of nitrogen application caused an increase in plant flowering due to the activation of gene expression involved in the flowering process 34-36 that explain the increase of inflorescences and flowers number after foliar application of A. maurorum extracts (Figures 5 and 6). Results also show a significant increase in the number of seeds after foliar spray with shoot and root extracts of A. maurorum compared to control except the spraying with 1.5 gL-1 of shoot extract (Figure 7). Increasing the number of F. vulgare seeds may be related to A. maurorum extract content of flavonoids, elements, carbohydrates, and vitamins 22, 37 that has a remarkable role in vegetative and floral growth enhancement of F. vulgare, resulting in an increase in seeds number compared to control plants.
CONCLUSIONS
Foeniculum vulgare, or fennel, is a plant used in diet, cooking, and medicine due to its flavor and health benefits. In the current study, exciting results show that spraying with shoot and root extract of Alhagi maurorum or camel thorn enhanced the vegetative, floral, and seed characteristics of F. vulgare. Most active compounds in the shooting part are present in roots except essential oils, which may cause a little difference in fennel plant growth with individual treatment of shoot extract. However, the interaction between the two factors enhanced the features of the study, especially the treatment with 3 g L-1 of shoot extract and 1.5 g L-1 of root extract, which achieved the highest number of seeds per plant.
Author Contributions: Conceptualization, W.H.N. and F.A.H., methodology W.H.N. and F.A.H., formal analysis W.H.N., writing original draft W.H.N. and updating draft F.A.H.
Funding: This research received no external funding.
Data Availability Statement: Data Availability Statements in the «Bionatura Research Data Policies» section at https://www.revistabionatura.com/policies.html.
Acknowledgments: We thank Hiba Asaad Majeed, an Agriculture College / University of Basrah undergraduate student.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Rather MA, Dar BA, Sofi SN, Bhat BA, Qurishi MA. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arabian Journal of Chemistry. 2016;9:S1574-S83.
2. Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674-.
3. Barros L, Carvalho AM, Ferreira IC. The nutritional composition of fennel (Foeniculum vulgare): Shoots, leaves, stems and inflorescences. LWT-Food Science and Technology. 2010;43(5):814-8.
4. Badgujar SB, Patel VV, Bandivdekar AH. <i>Foeniculum vulgare</i> Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed Res Int. 2014;2014:842674.
5. Al-Zubaidy, N. .; Al-Mubarak, N. F. .; Ahmed, A. M. . The Effect Of Fertilization And Repeated Mowing On Some Vegetative Characteristics And Yield Of Panicum Mombasa Plant. JLSAR 2021, 2, 34–45.
6. Damjanović B, Lepojević Ž, Živković V, Tolić A. Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: Comparison with hydrodistillation. Food Chemistry. 2005;92(1):143-9.
7. Fang L, Qi M, Li T, Shao Q, Fu R. Headspace solvent microextraction-gas chromatography–mass spectrometry for the analysis of volatile compounds from Foeniculum vulgare Mill. Journal of pharmaceutical and biomedical analysis. 2006;41(3):791-7.
8. Thabit S S, Awad M M, Abdulateef S M. Effects of In-Ovo Injection of sialic acid on Chick’s embryonic development and physiological traits. Revis Bionatura 2023;8 (2) 49. http://dx.doi.org/10.21931/RB/2023.08.02.49.
9. Roby MHH, Sarhan MA, Selim KA-H, Khalel KI. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Industrial crops and products. 2013;44:437-45.
10. Araque M, Rojas L, Usubillaga A. Antibacterial activity of essential oil of Foeniculum vulgare miller against multiresistant gram-negative bacilli from nosocomial infections. Science. 2007;15(3):366-70.
11. Thakur N, Sareen N, Shama B, Jagota K. Studies on in vitro antifungal activity of Foeniculum vulgare Mill. against spoilage fungi. Global Journal of Bio-Science and BioTechnology. 2013;2(3):427-30.
12. Taie H, Helal M, Helmy W, Amer H. Chemical composition and biological potentials of aqueous extracts of fennel (Foeniculum vulgare L). Journal of Applied Sciences Research. 2013;9(3):1759-67.
13. Kataoka H, Horiyama S, Yamaki M, Oku H, Ishiguro K, Katagi T, et al. Antiinflammatory and an-ti-allergic activities of hydroxylamine and related compounds. Biological and Pharmaceutical Bulletin. 2002;25(11):1436-41.
14. Choi E-M, Hwang J-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75(6):557-65.
15. Faudale M, Viladomat F, Bastida J, Poli F, Codina C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. Journal of agricultural and food chemistry. 2008;56(6):1912-20.
16. Albert-Puleo M. Fennel and anise as estrogenic agents. Journal of Ethnopharmacology. 1980;2(4):337-44.
17. Agarwal R, Gupta SK, Agrawal SS, Srivastava S, Saxena R. Oculohypotensive effects of Foeniculum vulgare in experimental models of glaucoma. Indian journal of physiology and pharmacology. 2008;52(1):77-83.
18. Ibraheem M W, Muhaimeed A R, Mohammed Th. T. Leg cuts from Awaasi lambs fed a diet with varying levels of Rhus coriaria L., Physical dissection and chemical composition. Revis Bionatura 2022;7(4) 4. http://dx.doi.org/10.21931/RB/2022.07.04.4.
19. Birdane FM, Cemek M, Birdane YO, Gülçin İ, Büyükokuroğlu ME. Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World Journal of Gastroenterology: W.J.G. 2007;13(4):607.
20. M. K. Rozbiany, P., M. Taha, S. Response Of Local Sour Orange (Citrus Aurantium L.) Seedlings To Foliar Application With Two Types Of Bio Stimulators. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 87-94. doi: 10.32649/ajas.2023.179718.
21. Abou El-Soud N, El-Laithy N, El-Saeed G, Wahby M, Khalil M, Morsy F, et al. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Macedonian J Med Sci. 2011;4(2):139-46.
22. Al-Snafi A. Alhagi maurorum as a potential medicinal herb: An Overview. International Journal of Pharmacy Review and Research. 2015;5:130-6.
23. Muhammad G, Hussain MA, Anwar F, Ashraf M, Gilani A.H. Alhagi: a plant genus rich in bioactives for pharmaceuticals. Phytotherapy research. 2015;29(1):1-13.
24. Mostafa RM, Essawy HS. Assessment of camel thorn (Alhagi maurorum) as new sources of bioactive compounds using GC-MS technique. A.P.R.I.L. 2019. 2019.
25. Ismail A, Sabra F. Allelopathic assessment of aqueous extract of Alhagi maurorum and Sorghum bicholor. Egy J Plant Pro Res. 2014;2:56-73.
26. Khalil R, Yusuf M, Bassuony F, Gamal A, Madany M. Phytotoxic effect of Alhagi maurorum on the growth and physiological activities of Pisum sativum L. South African Journal of Botany. 2020;131:250-8.
27. Mostafa R, Essawy H. Assessment of camel thorn (Alhagi maurorum) as new sources of bioactive compounds using GC-MS technique. Plant Omics. 2019:70-7.
28. Antonova G, Varaksina T, Zheleznichenko T, Stasova V. Changes in phenolic acids during maturation and lignification of Scots pine xylem. Russian journal of developmental biology. 2012;43(4):199-208.
29. Chattha F, Nisa M, Munawer M, Kousar S. Coumarin‐Based Heteroaromatics as Plant Growth Regulators. 2016.
30. Ainsworth EA, Bush DR. Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011;155(1):64-9.
31. Ghorbani Dehkordi A, Mashayekhi K, Kamkar B. Effect of Foliar Application of Sucrose, Boron, Po-tassium Nitrate and Salicylic Acid on Yield and Yield Components of Tomato var. Super A. Research in Crop Ecosystems. 2015;2(1):43-52.
32. Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial sym-biosis. Annual review of genetics. 2011;45(1):119-44.
33. Peck MC, Fisher RF, Long SR. Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. Journal of bacteriology. 2006;188(15):5417-27.
34. Yuan S, Zhang Z-W, Zheng C, Zhao Z-Y, Wang Y, Feng L-Y, et al. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proceedings of the National Academy of Sciences. 2016;113(27):7661-6.
35. Peng M, Bi YM, Zhu T, Rothstein SJ. Genome-wide analysis of Arabidopsis responsive transcriptome to nitrogen limitation and its regulation by the ubiquitin ligase gene NLA. Plant molecular biology. 2007;65(6):775-97.
36. Ali, H.H., AL-Rawi, K., Khalaf, Y., Alaaraji, S., Aldahham, B., Awad, M., Al-ani, O., Al-ani, F., Ali, A.T. Serum Caveolin-1 Level is Inversely Associated with Serum Vaspin, Visfatin, and HbA1c in Newly Diagnosed Men with Type-2 Diabetes (2022) Reports of Biochemistry and Molecular Biology, 11 (2), pp. 299-309.
37. Mohammed RK, Abd-alkadhemand NAD. Determination of Active Phytochemical Compounds of Alhagi Maurorum using Gas Chromatography-Mass Spectroscopy (GC-MS). Iraqi Journal of Science. 2022;63(1):87-97.
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Neamah, W.; Hasan, F. Effect of Foliar Application of Alhagi maurorum Extract on Foeniculum vulgare Growth. Revis Bionatura 2023;8 (4) 50. http://dx.doi.org/10.21931/RB/2023.08.04.50



















