Vol 8 No 4 2023 – 32
Nucleic acid amplification testing (NAT) impact on blood safety compared to Immunoassays in blood banks: A Review
Santiago Cadena-Ullauri1, Anibal Gaviria2,3, Patricia Guevara-Ramirez1, Viviana A. Ruiz-Pozo1, Rafael Tamayo-Trujillo1, Elius Paz-Cruz1, Ana Karina Zambrano1*.
1 Centro de Investigación Genética y Genómica, Facultad de Ciencias de la Salud Eugenio Espejo, Universidad UTE, Quito, Ecuador; santiagoa.cadena@ute.edu.ec, alexandra.guevara@ute.edu.ec, viviana.ruiz@ute.edu.ec, victor.tamayo@ute.edu.ec, elius.paz@ute.edu.ec, anazambrano17@hotmail.com,
2 Laboratorio de Genética Molecular, Centros Médicos Especializados Cruz Roja Ecuatoriana, Quito, Ecuador; anibalgaviria@hotmail.com.
3 Hemocentro Nacional, Cruz Roja Ecuatoriana, Quito, Ecuador; anibalgaviria@hotmail.com.
*Correspondence: anazambrano17@hotmail.com; Tel.: (02) 2 990 800
Available from: http://dx.doi.org/10.21931/RB/2023.08.04.33
ABSTRACT
Blood transfusions are fundamental to clinical procedures; however, many people worldwide cannot access safe blood. Blood product safety must be essential in each country’s national health policies. Several aspects of the blood donation process are carefully performed, including laboratory testing comprising blood type determination, antigen-antibody analyses, and nucleic acid amplification testing (NAT); however, NAT is not mandatory in all countries. The traditional screening method is based on antigen-antibody binding techniques, such as ELISA (enzyme-linked immunosorbent assay), with high sensitivity and specificity. Nevertheless, these methods have a seroconversion window period (WP), in which antigen-antibody testing cannot detect the pathogen and has not caused any symptoms yet. NAT is a sensitive molecular method based on viral nucleic acid amplification and detection. Moreover, its use in blood banks is increasing worldwide because it narrows the window period. For example, Huang et al. in 2017 reported the detection of 22 samples reactive only by nucleic acid testing for either HIV, HBV, or HCV compared with ELISA.
The present article shows how blood safety has improved by implementing NAT as a routine method for viral nucleic acid detection, highlighting the importance of this technique as evidenced by the findings presented herein. Moreover, these results are highly significant, demonstrating the relevance of NAT and advocating for its application on a global scale in blood management protocols. This development could be particularly beneficial for regions with a high viral infection prevalence, including many countries.
Keywords: Nucleic acid amplification, Immunoassay, viral infection, blood bank.
INTRODUCTION
Blood transfusions are fundamental to clinical procedures; however, many people worldwide, especially in low-income countries, cannot access safe blood 1–3. Blood product safety must be ensured in each country’s national health policies 4,5. Blood safety starts with a pre-donation survey to identify risky behaviors that could lead to viral or bacterial infections, endangering the people receiving the blood transfusion 4,6,7. Similarly, several aspects of the blood donation process should be carefully performed, including blood collection, labeling and handling each blood component, blood transfusion, and laboratory testing 4,7,8. Laboratory testing is comprised of blood type determination, antigen-antibody analyses, and nucleic acid amplification testing (NAT); however, in all countries, NAT is mandatory 1. For instance, in Latin America, Nicaragua, Costa Rica, Venezuela, and Uruguay do not perform NAT on blood donations.
The World Health Organization (WHO) promotes efforts to improve screening methods worldwide 4. The traditional screening method is based on antigen-antibody binding techniques, such as ELISA (enzyme-linked immunosorbent assay), CLIA (chemiluminescence immunoassay), or ECLIA (electrochemiluminescence immunoassay), which have high sensitivity and specificity, ranging from 95% to 99%, and from 90% to 99%, respectively 9–12. Nevertheless, these methods have a seroconversion window period (WP), which varies according to the virus and the patient 13,14. A window period refers to the early stages of an infectious disease, in which antigen-antibody testing cannot detect the pathogen and has not caused any symptoms yet. However, the host could be infectious and transmit the disease; most transfusion transmission infections occur because of WP 13–18. For example, in Germany before NAT, 1,500 hemophiliacs were infected with HIV in 1993 due to transfusion transmission infections. Similarly, in the USA, the situation was very similar, with 10,000 people infected by contaminated blood products 9.
NAT is a sensitive molecular method that could be used for viral or bacterial nucleic acid amplification and detection. Moreover, its use in blood banks is increasing because it narrows the window period of HIV, HBV, and HCV infections, providing an extra layer of safety 9,18,19. However, NAT also has limitations; for instance, NAT reactions are performed in pools; therefore, if a pool is reactive, each sample must be processed individually. Furthermore, NAT requires specialized infrastructure, consumables, and equipment.
The present review describes the screening methods used in blood banks, including immunoassays and NAT, to detect the presence of viruses in blood products. Moreover, it highlights the importance of NAT implementation by describing studies that have reported infections only detected by NAT. In conclusion, NAT reduces the window period, detects occult infections, and ultimately increases blood safety.
IMMUNOASSAYS
Immunoassays are used in many clinical settings; their use includes the detection of antigens, autoantibodies, tumor markers, hormone levels, drugs, and antibodies against pathogens, such as viruses or bacteria. Immunoassays detect the concentration or presence of a molecule by using an antigen or antibody for its detection 20–22. In humans, for example, the immune system can generate a response to a foreign body; they do so by synthesizing proteins (antibodies) that will recognize the invader (antigen) 20,23,24. In this technique, those antibodies generated in the immune response will be detected.
There are five classes of antibodies, depending on their structure and biological function: Immunoglobulin (Ig) G, IgA, IgE, IgM, and IgD; among these, IgG is the antibody with the highest availability and concentration 25. The structure of IgG is presented in Figure 1. The antibody is composed of two heavy chains and two light chains. Moreover, there are two main regions, the Fab region that is specific and will bind to the antigen, and the Fc region that interacts with cell surface receptors 20,26.
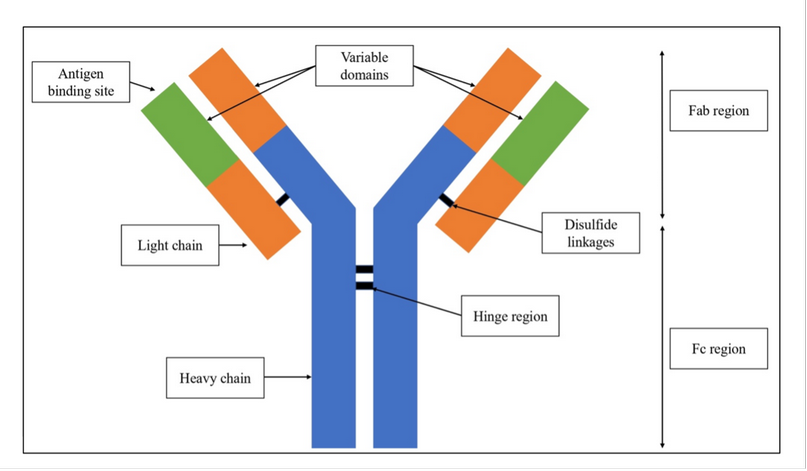
Figure 1. Immunoglobulin G structure.
Some factors that could interfere with the test include contamination, binding affinity, quality of the wells, buffer pH, and cross-reactivity 27,28. However, enzyme-linked immunosorbent assay (ELISA) is considered the gold standard of immunoassays 29; it is used to detect and quantify proteins, hormones, glycoproteins, antibodies, and antigens. ELISAs require 96-well polystyrene plates, primary or secondary antibodies, antigen/analyte, enzymes, and chromogen/substrate 30.
Enzyme-linked immunosorbent assay (ELISA)
ELISAs steps, generally, are 30,31: First, antigen or antibody plate coating, followed by the addition of the samples. The next step is blocking, usually with bovine serum albumin (BSA). Then, the detection by using conjugated antibodies and substrates, typically horseradish peroxidase (HRP) or alkaline phosphatase (AP). Finally, the results are read by spectrophotometry to determine the presence of the analyte of interest. Moreover, there are different types of ELISAs. The major ELISA types are direct, indirect, sandwich, and competitive 31.
Direct ELISA
This type of test (Figure 2. A) starts by adding the sample to the wells in the plate, where the antigen of interest will bind. Next, blocking and washing steps are followed by adding an enzyme-conjugated primary antibody, which will bind to the antigen of interest. Then, a substrate is added, and finally, the reaction is detected, displaying the results. The analyte concentration is proportional to the intensity of the signal. The disadvantage of this technique is a lower sensitivity compared to other types of ELISAs 32; however, one advantage is that it only requires one antibody, which eliminates the secondary antibody cross-reactivity 30,31.
Indirect ELISA
The indirect ELISA (Figure 2. B) is very similar to a direct ELISA; the main difference is that indirect ELISA requires two antibodies 33. The primary antibody will bind directly to the protein of interest, and a secondary antibody will be used to detect the primary antibody. The secondary antibody is usually conjugated with an enzyme that hydrolyzes or oxidates the substrate, resulting in a color change 33. The main disadvantage of this technique is the risk of cross-reactivity; however, the advantages are lower costs and higher sensitivity 30,31.
Sandwich ELISA
Two antibodies will be used in this type of ELISA (Figure 2. C). A capture antibody and an enzyme-conjugated antibody. In the first step of this technique, the plate is coated with a capture antibody. Then, the sample containing the proteins of interest is added. The next step involves using an enzyme-conjugated antibody that will bind to the proteins. The process ends with adding a substrate, resulting in a color change. The disadvantage of this technique is the high cost and time; however, this type of ELISA has the most heightened sensitivity 30,31.
Competitive ELISA
Antibodies will compete for antigen binding in the competitive ELISA (Figure 2. D). In the first step, the plates are coated with an antigen. Then, the sample will be tested to determine if it contains the antibodies of interest, and an enzyme-conjugated antibody will be added to the plates; both antibodies will compete for antigen binding. The concentration of each antibody will determine which antibody will ‘win’ the competition. If the sample has the highest concentration, no color change will be detected; however, if the enzyme-conjugated antibody has the highest concentration, a color change will occur. The disadvantage of this technique is its low specificity, whereas an advantage is that it offers low variability and multiple antigens could be used 30,34.
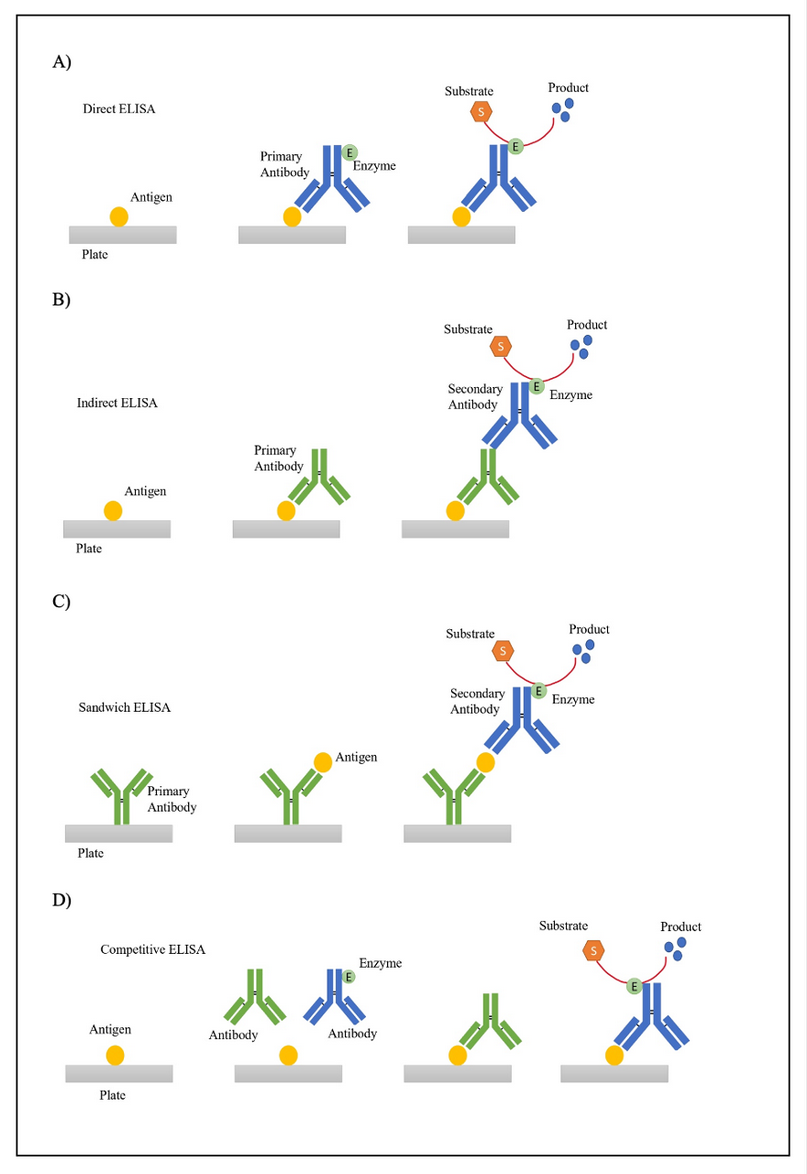
Figure 2. Overview of different ELISA types A) Direct ELISA B) Indirect ELISA C) Sandwich ELISA D) Competitive ELISA.
Chemiluminescent immunoassay (CLIA)
CLIA is an assay developed to determine the analyte’s concentration on a sample using the luminescence intensity of a chemical or enzymatic reaction 12,21. Luminescence is the emission of visible radiation with wavelengths of 300-800nm 35. CLIA offers several advantages, including high specificity and sensitivity, simple equipment, short time, low cost, simplicity, and high throughput 36,37. CLIA is similar to ELISA, as it is also based on immunoreactions. However, the main difference is that in CLIA, the results are measured as absolutes according to luminescence intensity, while in ELISA, the results are relative and determined from the substrate’s color change 35,36.
Different types of CLIA exist, including direct and indirect approaches, which could be competitive or non-competitive. The direct method uses luminophore markers such as ruthenium and acridinium esters, whereas the indirect system enzyme markers like alkaline phosphatase and horseradish peroxidase utilize luminol as substrate (Figure 3) 35,38.
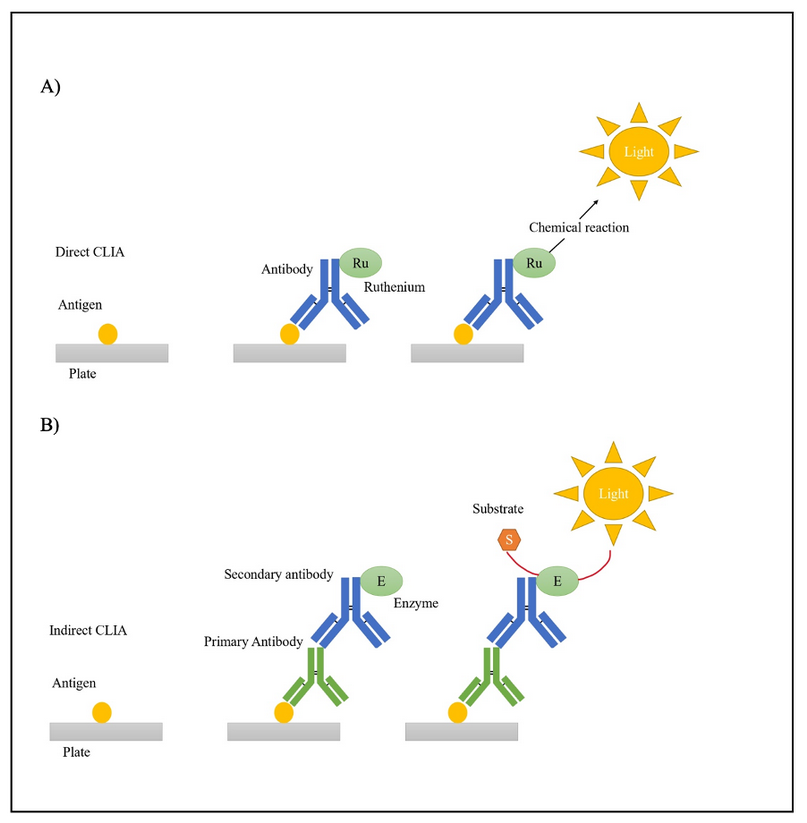
Figure 3. A) Direct CLIA and B) Indirect CLIA overview.
Nucleic acid amplification testing (NAT):
Nucleic acid testing is a molecular technique for viral nucleic acid detection with sensitivity and specificity values ranging from 92.5% to 100% and from 99.8 to 100%, respectively, 39–41. The basis of this technique consists of the amplification and detection of viral RNA or DNA 9,42,43. One example of a NAT assay is the reverse transcription-quantitative polymerase chain reaction (RT-qPCR), which starts with retrotranscribing the viral RNA into cDNA, followed by several cycles of denaturation, primer annealing, and extension. Lastly, the fragments of interest are detected and quantified (Figure 4) 44. Furthermore, NAT offers several advantages compared with other screening methods, such as ELISA and CLIA; these include minimizing the risk of contamination, the possibility of multiplexing, identifying different viruses, and reducing the window period 9,45–47. NAT’s impact on blood safety is significant; for instance, Roth et al. (2008) reported that out of more than 300 million donations, 2,808 virus-contaminated donations were identified by NAT only 9. Moreover, NAT could also be developed and used to detect other viruses, such as West Nile Virus, the SARS-CoV-2 48,49, and emerging viruses; the only requirement would be to identify the viral genome sequence 9.
Limitations of this approach include that NAT reactions are performed in pools of different numbers, for example, 96 samples per reaction. If a pool is reactive, each sample will be processed individually, increasing the cost and time of the process. Furthermore, NAT requires specialized infrastructure, consumables, and equipment. Moreover, studies have found that pool NAT could miss infections with low viral loads, which could only be detected in individual tests 39,50.
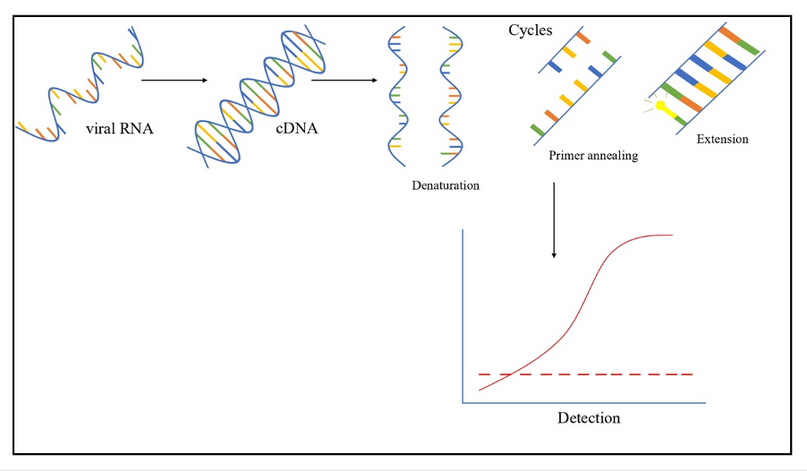
Figure 4. RT-qPCR overview.
Comparison between NAT and immunoassays for viral detection
HIV
In HIV, the window period in antigen/antibody testing could be 18 to 28 days, whereas, in NAT, the window period shortens by 8 to 20 days (Figure 5) 16. For example, in 2017, Huang, W. et al. compared ELISA and NAT methods in a hospital in China between 2015 and 2016, where for HIV, they found 605 cases of NAT(+) and Immunoassay (+) 16. However, they also found 21 cases of NAT(+) and Immunoassay(-), followed up on one of the cases and when testing one week later, found that it was a window period case. In addition, 143 cases of NAT(-) and Immunoassay (+) were found, possibly due to false positives and insufficient ELISA specificity 16. Furthermore, Table 1 presents more studies with similar cases of window period infections.
HCV
In HCV, the window period could range from 20 to 36 days 15,51. In contrast, by using NAT, the viral RNA can be detected as soon as two days after exposure (Figure 5) 51,52. For example, Hourfar et al. in 2008 analyzed the performance of NAT vs. serological methods. They found that 23 donations were NAT-reactive only for HCV, which could be attributed to an infection in the window period phase 53. Similarly, Velati, C. et al. reported in 2008 the detection of 27 NAT(+) CLIA(-) donations out of 10,776,228 units. During a follow-up, it was found that the infections in all 27 patients were in the window period, and later, NAT(+) CLIA(+) 17 was tested. Similarly, Stramer et al. 2004 found that 170 donations, out of approximately 40 million, were reactive only for NAT 54. Table 1 presents more studies comparing NAT and Immunoassays.
HBV
HBV’s window period can range from 30 to 50 days 55. In addition, there is a risk of an occult hepatitis B infection with undetectable HBsAg levels 56. On the other hand, by using NAT, the virus can be detected within one week of infection, thus shortening the window period. Moreover, NAT can detect the presence of the virus even in its hidden infection state (Figure 5) 55. For example, Keechilot, et al. in 2016 analyzed 24,338 donations, 24,214 of these were negative for all serological markers (HIV, HBV, HCV, malaria, and syphilis). However, 5 NAT-only reactive samples for HBV were identified. The authors attributed this to occult or window period infections 56. Similarly, Hourfar, et al. in 2008 reported that out of 31,524,571, 22 samples were reactive only for NAT, indicating infections during their window period 53. Minegishi, et al. in 2003 conducted a study on over 11 million samples and identified that out of 181 HBV-NAT-positive samples, 172 were negative by immunoassays 57. Similar studies are presented in Table 1.
Moreover, the sensitivity and specificity of ELISA tests can be determined through a comparative analysis of the studies mentioned above and using NAT as the gold standard. The sensitivity stands at 92%, whereas the specificity is 99%. Even though these values are relatively high, infections may not be detected, which could lead to severe health consequences.
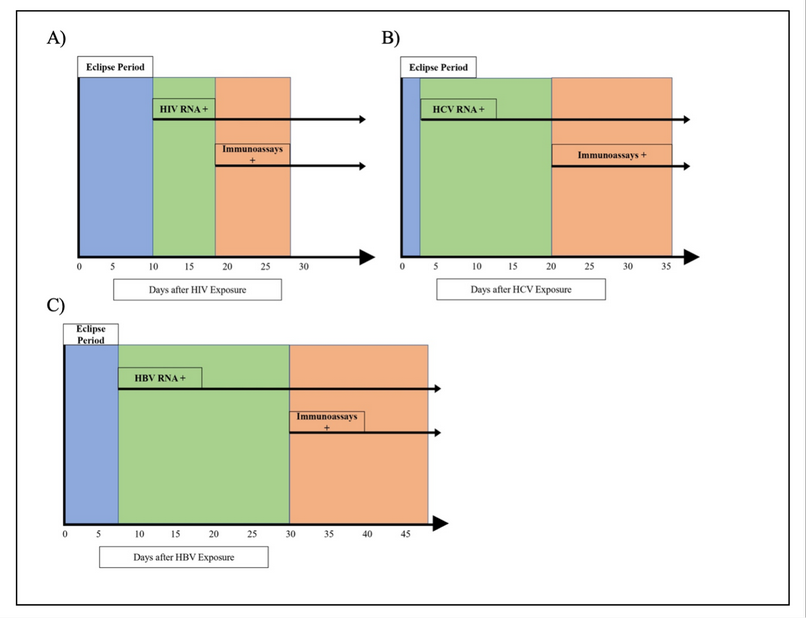
Figure 5. Detection periods comparison between NAT and Immunoassays for A) HIV. B) HCV and C) HBV.
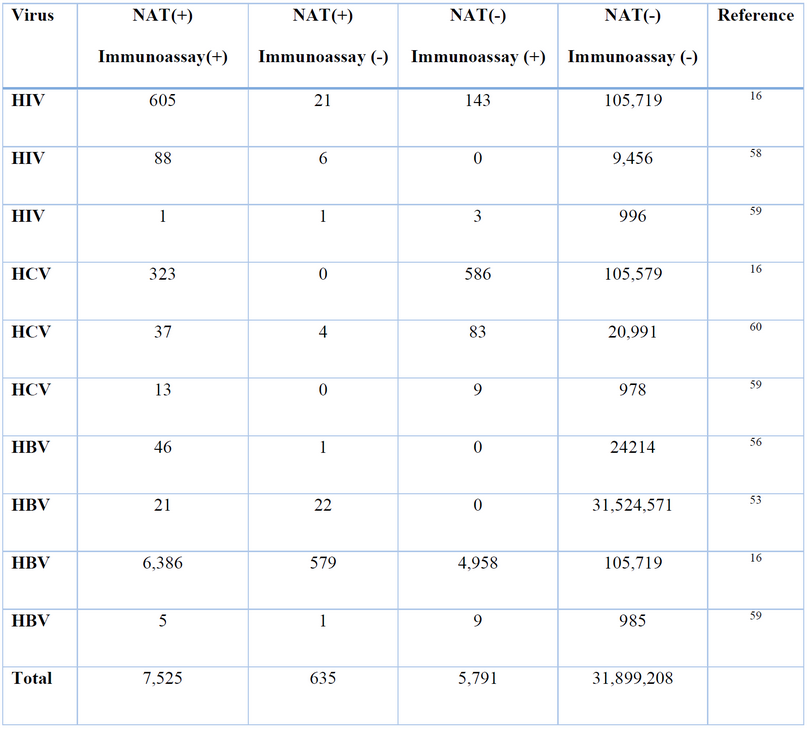
Table 1. NAT vs Immunoassay comparison for viral detection.
DISCUSSION
Lives are saved daily thanks to blood transfusions, and this process has become a fundamental part of clinical procedures; hence, ensuring blood safety is essential, and countries must develop public health policies to provide safe blood to everyone 4,5. Moreover, the blood donation process has changed over time, and nowadays, several steps and procedures increase blood safety. These include a thorough standardized questionnaire to identify potential risks, serological tests to determine blood type, irregular antibodies, and infections, and molecular tests for viral nucleic acid detection 4,7,8.
One of the main issues regarding blood safety is the possible presence of infections in the WP phase. In this phase, the presence of the pathogen cannot be detected by serological testing because the body has not had enough time to produce detectable antibodies. However, the donated blood could be infected, affecting the recipient 13,14,18. In this regard, NAT is a molecular technique that can reduce the WP; for instance, for HIV, the serological WP is from 18 to 45 days, and by using NAT, the detection could be performed as soon as 11 days after exposure, reducing the WP by 7 days; increasing the capacity to detect viral infections and reducing the risk of transfusion-transmitted infections 9,18,19.
The present article shows how blood safety has improved by implementing NAT as a routine method for viral nucleic acid detection, highlighting the importance of this technique as evidenced by the findings presented herein. Moreover, these results are highly significant, demonstrating the relevance of NAT and advocating for its application on a global scale in blood management protocols. This development could be particularly beneficial for regions with a high viral infection prevalence, including many countries.
CONCLUSIONS
In conclusion, nucleic acid testing (NAT) is a powerful technique capable of detecting viral nucleic acids, even when immunoassays cannot; thus, it narrows the window period. Moreover, NAT can detect occult HBV infections. For example, only in the studies mentioned, NAT has prevented 1,923 hemo components (red blood cells, platelets, and plasma) infected with HIV, HCV, or HBV from being transfused. The novelty of the present research lies in its comprehensive review of the current literature, which describes the sensitivity and specificity of the methods used in blood banks and focuses on the importance of NAT implementation. We aim that countries that have not yet applied this technology will realize its significance in providing safe blood for everyone.
Supplementary Materials: The following are available online at www.revistabionatura.com/xxx/s1, Figure S1: title, Table S1: title, Video S1: title.
Author Contributions: Conceptualization, SCU, AKZ, AG; writing—original draft preparation, SCU; writing—review and editing, AKZ, AG, EPC, RTT, PGR, VARP; project administration, AKZ. All authors have read and agreed to the published version of the manuscript.”
Funding: No funding was received.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Acknowledgments: The authors thank Universidad UTE and Cruz Roja Ecuatoriana for their support.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. World Health Organization. Blood safety and availability [Internet]. 2022. p. 2–9. Available from: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability(Accessed:02/05/2021)%0Ahttp://www.who.int/mediacentre/factsheets/fs279/en/
2. Jenny HE, Saluja S, Sood R, Raykar N, Kataria R, Tongaonkar R, et al. Access to safe blood in low-income and middle-income countries : lessons from India. 2017;1–6.
3. Yonemura S, Doane S, Keil S, Goodrich R, Pidcoke H, Cardoso M. Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology. Blood Transfus. 2017;15:357–64.
4. Harris JC, Crookston KP. Blood product safety. Vol. 3, StatPearls Publishing. 2022.
5. Weimer A, Tagny CT, Tapko JB, Gouws C, Tobian AA., Ness PM, et al. Blood transfusion safety in sub-Saharan Africa: a literature review of changes and challenges in the 21st century. Transfusion. 2019;59(January):412–27.
6. Centers for Disease Control and Prevention. Blood Safety Basics [Internet]. March 18, 2020. 2020. p. 2–4. Available from: https://www.cdc.gov/bloodsafety/basics.html
7. Godbey EA, Thibodeaux SR. Seminars in Hematology Ensuring safety of the blood supply in the United States : Donor screening , testing , emerging pathogens , and pathogen inactivation ✩. 2019;56:229–35.
8. Leparc GF. Safety of the Blood Supply. Cancer Control. 2015;22:7–25.
9. Roth WK. History and Future of Nucleic Acid Amplification Technology Blood Donor Testing. Transfus Med Hemotherapy. 2019;46(2):67–75.
10. Jagani R, Dimr U, Kumar S, Pawar A. Experience of Individual Donor Nucleic Acid Testing on Screening of Blood Donors for Human Immunodeficiency Virus, Hepatitis C Virus, and Hepatitis B Virus at an Apex Blood Bank of Northern India. Med J Dr D Y Patil Vidyapeeth. 2022;15(1):49–53.
11. Candotti D, Laperche S. Hepatitis B virus Blood Screening : Need for Reappraisal of Blood Safety Measures? Front Med. 2018;5(February):1–10.
12. Chang L, Zhao J, Guo F, Ji H, Zhang L, Jiang X, et al. Comparative Evaluation and Measure of Accuracy of ELISAs, CLIAs, and ECLIAs for the Detection of HIV Infection among Blood Donors in China. Can J Infect Dis Med Microbiol. 2020;2020.
13. Quinn B, Pearson R, Cutts J, Seed C, Scott N, Hoad V, et al. Blood donation amongst people who inject drugs in Australia: research supporting policy change. Vox Sang. 2020;115(3):162–70.
14. Grubyte S, Urboniene J, Nedzinskiene L, Jelinskaite A, Zagminas K, Ambrozaitis A, et al. Prevalence, incidence and residual risk of transfusion transmitted viruses (HBV, HCV and HIV infections) in Lithuanian blood donors from 2004 to 2018: The incidence/ window-period model study. PLoS One [Internet]. 2021;16(2 February 2021):1–16. Available from: http://dx.doi.org/10.1371/journal.pone.0246704
15. Gupta E, Bajpai M, Choudhary A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 2014;8(1):19–25.
16. Huang W, Wei W, Shi XT, Jiang T. The analysis of the detection performance of nucleic acid testing and ELISA for HIV, HBV and HCB. Front Lab Med [Internet]. 2017;1(4):200–2. Available from: https://doi.org/10.1016/j.flm.2017.12.004
17. Velati C, Romanò L, Fomiatti L, Baruffi L, Zanetti AR, Sciariada L, et al. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: A 6-year survey. Transfusion. 2008;48(10):2205–13.
18. Chigurupati P, Murthy KS. Automated nucleic acid amplification testing in blood banks: An additional layer of blood safety. Asian J Transfus Sci. 2015;9(1):9–11.
19. Wu D, Feng F, Wang X, Wang D, Hu Y, Yu Y, et al. The impact of nucleic acid testing to detect human immunodeficiency virus , hepatitis C virus , and hepatitis B virus yields from a single blood center in China with 10 ‑ years review. BMC Infect Dis [Internet]. 2022;1–10. Available from: https://doi.org/10.1186/s12879-022-07279-5
20. Ju H, Lai G, Yan F. Immunoassay Introduction. In: Immunosensing for Detection of Protein Biomarkers. 2017. p. 1–30.
21. Wang MY, Devare S, Liu JF, Lv XT, Yin P, Guo N, et al. Comparison of three immunoassay systems for screening of HIV infection in blood donation in China. Ann Blood. 2019;4(May 2016):13–13.
22. Zhao J, Zhao F, Han W, Xu X, Wang L, Li R, et al. HTLV screening of blood donors using chemiluminescence immunoassay in three major provincial blood centers of China. BMC Infect Dis. 2020;20(1):1–9.
23. Tay MZ, Wiehe K, Pollara J. Antibody dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10(FEB):1–18.
24. Murin CD, Wilson IA, Ward AB. Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nat Microbiol [Internet]. 2019;4(5):734–47. Available from: http://dx.doi.org/10.1038/s41564-019-0392-y
25. Thomson CA. IgG Structure and Function. In: Encyclopedia of Immunobiology [Internet]. Elsevier; 2016. p. 15–22. Available from: http://dx.doi.org/10.1016/B978-0-12-374279-7.05002-5
26. Rispens T, Davies AM, Ooijevaar-de Heer P, Absalah S, Bende O, Sutton BJ, et al. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic fab arm exchange. J Biol Chem [Internet]. 2014;289(9):6098–109. Available from: http://dx.doi.org/10.1074/jbc.M113.541813
27. Kim H, Chung DR, Kang M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst. 2019;144(8):2460–6.
28. Stevens KG, Pukala TL. Conjugating immunoassays to mass spectrometry: Solutions to contemporary challenges in clinical diagnostics. TrAC – Trends Anal Chem [Internet]. 2020;132:116064. Available from: https://doi.org/10.1016/j.trac.2020.116064
29. Tiwari AK, Upadhyay AP, Arora D, Wadhwa T, Aggarwal G, Pabbi S, et al. Head-to-head comparison of Enzyme Linked Immunosorbent Assay (ELISA) and Enhanced Chemiluminescence Immunoassay (ECLIA) for the detection of Transfusion Transmitted Disease (TTD) Markers; HIV, HCV and HBV in blood donors, in India. J Virol Methods [Internet]. 2020;285(August):113962. Available from: https://doi.org/10.1016/j.jviromet.2020.113962
30. Alhajj M, Farhana A. Enzyme Linked Immunosorbent Assay (ELISA). In: Molecular Biomethods Handbook: Second Edition. 2022. p. 657–82.
31. Drijvers JM, Awan IM, Perugino CA, Rosenberg IM, Pillai S. The Enzyme-Linked Immunosorbent Assay: The Application of ELISA in Clinical Research [Internet]. Basic Science Methods for Clinical Researchers. Elsevier Inc.; 2017. 119–133 p. Available from: http://dx.doi.org/10.1016/B978-0-12-803077-6.00007-2
32. Wu J, Ju HX. Clinical immunoassays and immunosensing. Compr Sampl Sample Prep Anal Tech Sci. 2012;3:143–67.
33. Lin A V. ELISA: Methods and Protocols. In: ELISA: Methods and Protocols. 2015. p. 1–216.
34. Huang W, Wei W, Shi XT, Jiang T. The analysis of the detection performance of nucleic acid testing and ELISA for HIV, HBV and HCB. Front Lab Med [Internet]. 2017;1(4):200–2. Available from: https://doi.org/10.1016/j.flm.2017.12.004
35. Cinquanta L, Fontana DE, Bizzaro N. Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Autoimmun Highlights. 2017;8(1).
36. Azim MAU, Hasan M, Ansari IH, Nasreen F. Chemiluminescence Immunoassay: Basic Mechanism and Applications. Bangladesh J Nucl Med. 2018;18(2):171–8.
37. Lu Y, Zheng L, Houquan Z, Sijing L, Yaozong H, Yunlon G, et al. Application of chemiluminescence immunoassay method in HIV antibody/antigen detection and analysis of confirmationcases. J Clin Hematol. 2022;360–563.
38. Wang C, Wu J, Zong C, Xu J, Ju HX. Chemiluminescent Immunoassay and its applications. Fenxi Huaxue/ Chinese J Anal Chem [Internet]. 2012;40(1):3–10. Available from: http://dx.doi.org/10.1016/S1872-2040(11)60518-5
39. Hans R, Marwaha N. Nucleic acid testing-benefits and constraints. Asian J Transfus Sci. 2014;8(1):2–3.
40. Rocha D, De Melo GC, Carneiro JMH, Ribeiro M, Ribeiro S, De Godoy DT, et al. Use of a NAT-based assay to improve the surveillance system and prevent transfusion-transmitted malaria in blood banks. Malar J [Internet]. 2020;19(1):1–9. Available from: https://doi.org/10.1186/s12936-020-03345-y
41. Chiquete E, Sánchez L V., Becerra G, Quintero A, Maldonado M, Panduro A. Performance of the serologic and molecular screening of blood donations for the hepatitis B and C viruses in a Mexican Transfusion Center. Ann Hepatol Off J Mex Assoc Hepatol. 2005;4(4):275–8.
42. Kleinman SH, Busch MP. Assessing the impact of HBV NAT on window period reduction and residual risk. J Clin Virol. 2006;36:495–512.
43. Mabunda N, Augusto O, Zicai AF, Duajá A, Oficiano S, Ismael N, et al. Nucleic acid testing identifies high prevalence of blood borne viruses among approved blood donors in Mozambique. PLoS One. 2022;17(4 April):1–12.
44. Macchi B, Frezza C, Marino-Merlo F, Minutolo A, Stefanizzi V, Balestrieri E, et al. Appraisal of a simple and effective RT-qPCR assay for evaluating the reverse transcriptase activity in blood samples from HIV-1 patients. Pathogens. 2020;9(12):1–9.
45. Kurt Roth W, Schuller A, Busch MP, Reesink HW, Panzer S. International survey on NAT testing of blood donations: Expanding implementation and yield from 1999 to 2009. Vox Sang. 2012;102(1):82–90.
46. Jackson BR, Busch MP, Stramer SL, AuBuchon JP. The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion. 2003;43(6):721–9.
47. Safic Stanic H, Babic I, Maslovic M, Dogic V, Bingulac-Popovic J, Miletic M, et al. Three-Year Experience in NAT Screening of Blood Donors for Transfusion Transmitted Viruses in Croatia. Transfus Med Hemotherapy. 2017;44(6):415–20.
48. Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, et al. Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing. N Engl J Med. 2005;353(5):460–7.
49. CDC. Overview of Testing for SARS-CoV-2 | CDC. Centers for Disease Control and Prevention. 2020.
50. Baruah S, Pal L. Seven Years Experience in NAT Testing of Blood Donors in a Tertiary Care Centre. Int J Contemp Med Res [IJCMR]. 2019;6(7):4–7.
51. Fox R, Corcorran M, Spach D. Diagnosis of Acute HCV Infection. University of Washington. 2021. p. 1–13.
52. Busch MP, Page Shafer KA. Acute-phase hepatitis C virus infection: Implications for research, diagnosis, and treatment. Clin Infect Dis. 2005;40(7):959–61.
53. Hourfar MK, Jork C, Schottstedt V, Weber-Schehl M, Brixner V, Busch MP, et al. Experience of German Red Cross blood donor services with nucleic acid testing: Results of screening more than 30 million blood donations for human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus. Transfusion. 2008;48(8):1558–66.
54. Stramer SL, Glynn SA, Kleinman SH, Strong DM, Caglioti S, Wright DJ, et al. Detection of HIV-1 and HCV Infections among Antibody-Negative Blood Donors by Nucleic Acid–Amplification Testing. N Engl J Med. 2004;351(8):760–8.
55. Kuhns MC, Busch MP. New strategies for blood donor screening for hepatitis B virus: Nucleic acid testing versus immunoassay methods. Mol Diagnosis Ther. 2006;10(2):77–91.
56. Keechilot CS, Shenoy V, Kumar A, Biswas L, Vijayrajratnam S, Dinesh K, et al. Detection of occult hepatitis B and window period infection among blood donors by individual donation nucleic acid testing in a tertiary care center in South India. Pathog Glob Health [Internet]. 2016;110(7–8):287–91. Available from: http://dx.doi.org/10.1080/20477724.2016.1248171
57. Minegishi K, Yoshikawa A, Kishimoto S, Yugi H, Yokoya N, Sakurada M, et al. Superiority of minipool nucleic acid amplification technology for hepatitis B virus over chemiluminescence immunoassay for hepatitis B surface antigen screening. Vox Sang. 2003;84(4):287–91.
58. Krajden M, Cook D, Mak A, Chu K, Chahil N, Steinberg M, et al. Pooled nucleic acid testing increases the diagnostic yield of acute HIV infections in a high-risk population compared to 3rd and 4th generation HIV enzyme immunoassays. J Clin Virol [Internet]. 2014;61(1):132–7. Available from: http://dx.doi.org/10.1016/j.jcv.2014.06.024
59. Ebeid EY, Kholeif HAE, Hussein NH. Role of Nucleic Acid Test (NAT) in Detection of Transfusion Transmitted Viruses in Comparison to Other Methods. Egypt J Hosp Med. 2019;76(2):3542–9.
60. Arora S, Doda V. Role of signal-to-cut-off ratios of anti-hepatitis C virus antibody by enzyme immunoassays along with ID-NAT for screening of whole blood donors in India. Asian J Transfus Sci. 2016;10(1):75–8.
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Cadena-Ullauri, S.; Gaviria, A.; Guevara-Ramirez, P.; Ruiz-Pozo, VA.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Zambrano, AK. Nucleic acid amplification testing (NAT) impact on blood safety compared to Immunoassays in blood banks: A Review. Revis Bionatura 2023;8 (4) 31. http://dx.doi.org/10.21931/RB/2023.08.04.33



















