Vol 8 No 4 2023 – 17
Assessment of the microbiological quality and heavy metal risk in vegetable species marketed in outdoor fairs
1 Universidad Politécnica Salesiana, Carrera de Ingeniería en Biotecnología de los Recursos Naturales, Sede Quito, Campus El Girón, Grupo de Investigación y Desarrollo en Ciencias Aplicadas a los Recursos Biológicos, Av. 12 de octubre N2422 y Wilson, Quito, 170109, Ecuador; ecoyagoc@ups.edu.ec.
2 Universidad Politécnica Salesiana, Carrera de Ingeniería en Biotecnología de los Recursos Naturales, Grupo de Investigación en Biotecnología Aplicada a los Recursos Naturales, Av. 12 de octubre N2422 y Wilson, Quito, 170109, Ecuador; racurio@ups.edu.ec, gmendez@ups.edu.ec.
* Correspondence: ecoyagoc@ups.edu.ec; Tel.:+593(995129321) (Quito, Ecuador)
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.18
ABSTRACT
The objective of this study was to evaluate the possible health risks associated with the content of heavy metals and microorganisms in the consumption of watercress, lettuce, lemon balm and taraxaco. The products were purchased in several markets in the city of Quito. In this study, the products marketed in the north and center of the town showed better size characteristics, while the soluble solids, pH and ash showed no definite behavior. In addition, due to the volcanic origin of the Ecuadorian soils in which the samples under study were grown, it could influence the lead content that varied between 0.02 ppm (taraxacum, lemon balm and lettuce) up to 2.60 ppm (watercress). This value exceeded the maximum limit allowed by the CODEX, becoming a health risk. In addition, the microbial quality of the different species under study showed high contamination with mesophilic aerobic microorganisms and parasites. These results provide valuable information that could finance food safety decisions by the government to protect health and improve the quality of life of the people of Quito.
Keywords: food safety, mesophilic aerobic, parasites, lead, Cadmium
INTRODUCTION
Vegetables are critical dietary components because they provide essential nutrients, such as vitamins, minerals and fibers, and many health benefits. Food safety has been affected since most vegetables absorb contaminants in the soil and accumulate in different foliar tissues or cell walls 1. The risk of microbiological contamination on leafy greens is concerning.
In the United States (US) in 2009, leafy greens were identified at the top of the 10 riskiest foods regulated by the Food and Drug Administration (FDA) 2, with 22% of foodborne illnesses associated with leafy greens consumption 3, and involved with significant economic losses.
Fresh vegetables and fruits can be contaminated with pathogenic bacteria and heavy metals. During pre-harvest, contact with contaminated irrigation water, soil, fecal matter of wild animals, and communication with asymptomatic human carriers can contaminate the products. At the postharvest level, the products are contaminated by contact with contaminated water, other asymptomatic human carriers, or the production process and distribution environment 4.
To explain how microorganisms of concern are transmitted across the food chain, we studied risk factors contributing to microbial contamination of vegetables eaten raw in markets. Edible and medicinal plant species can be obtained at fairs or wholesale markets, selling products shown not to have microbiological and bromatological controls. In general, in products that are consumed fresh, it has been found that there is the possibility of more significant contamination of microorganisms that could cause certain diseases in humans 5.
The food pattern in Ecuador is linked to individual economic access, religion, and ancestral and current culture. 6, means of surveys indicate that a person in Ecuador consumes around 183 g/day between fruits and vegetables. Nevertheless, the population in these last years has been linked to their nutritional pattern in health care; this is why, according to the FAO, in 2016, there were 28307 tons of vegetables, a figure that has been increasing since several years ago, indicating that the consumption of vegetables has spread and therefore the market offering.
Watercress (Nasturtium officinale R. Br.) is one of the most consumed vegetables in Ecuador. It has been used since ancient times as traditional medicine to calm respiratory problems and skin diseases. According to 7, a watercress is a plant belonging to the cruciferous family that can grow spontaneously near almost all water courses; it will always require an acceptable flood level in fresh water. The watercress contains sodium, potassium, calcium, iron, sulfur, fiber, pro-vitamin A and vitamin C 8. On the other hand, it has been determined that the leaves and stems of the watercress may contain small slugs that, in turn, host parasites such as Fasciola hepatica.
The lettuce (Lactuca sativa L.) is another more frequently consumed vegetable. This provides a high content of carotenoids, vitamin C, minerals, fiber, and water. It also provides an alkaline reaction to the human body, accompanied by a high cellulose content and good-quality proteins 9. The most common diseases in cabbage lettuce are derived from inadequate handling in the postharvest and poor control of the optimum temperature in all the production processes. According to 10, among the microorganisms that may be present are bacteria (Coliforms, Pseudomonas spp. and Erwinia, Escherichia coli), parasites (Entamoeba histolytica, Giardia spp.), viruses (Norwalk, hepatitis A) and fungi (Botrytis, Fusarium) that form products that cause diseases in the human being.
Lemon balm (Melissa officinalis L.) and taraxaco (Taraxacum officinale F.H. Wigg.) grow exposed to highly interacting microorganisms that are in symbiosis or cause physiological alterations. The genera that are most interacting with these species are Agrobacterium spp., Clavibacter spp., Erwinia spp., Pseudomonas spp., Xanthomonas spp., Streptomyces spp., Xylella spp., and those that infect them through cross-contamination are: Clostridium botulinum, Staphylococcus aureus, Bacillus cereus and the family Enterobacteriaceae. Lemon balm and taraxaco are cultivated in the Ecuadorian inter-Andean region, which has the presence of heavy metals of a geographical nature. In addition, the recurrent agricultural activity increases the presence of metals due to using fertilizers, pesticides, manure, slurry and sewage sludge 11.
All the plant species already mentioned are part of a food and medicinal culture available to everyone. For this reason, the objective of this study was to evaluate the possible health risks associated with the content of heavy metals and microorganisms in the consumption of watercress, lettuce, lemon balm and taraxaco in the markets of the Metropolitan District of Quito to raise awareness of the quality and safety of these products that form a basis in Ecuadorian food.
MATERIALS AND METHODS
Reagents and Standards
Reagents of analytical grade as nitric acid (PumChem CID: 944), sulfuric acid (PumChem CID: 1118), sodium hydroxide (PumChem CID: 14798) and sodium chloride were obtained from Panreac (Barcelona, Spain). Cadmium, lead standards, cadmium standard and violet crystal were purchased from Merck (Labomersa S. A., Ecuador). Peptone was obtained from BD. (Biosciences, United States).
Plant Materials
The watercress (Nasturtium officinale R. Br.), lettuce (Lactuca sativa L.), lemon balm (Melissa officinalis L.) and taraxaco (Taraxacum officinale (L.) Weber ex F.H. Wigg) were purchased from an average of thirty markets of the zonal distributions of the Metropolitan District of Quito (Figure 1). The leaves were considered a sample unit, and a whole head of lettuce was regarded as one sample. Approximately 1 Kg of each species was purchased randomly from four retail sites within each market. The amount of samples was selected as described by the INEN-1750 12. After sampling, all samples were placed in sterile zipped bags and immediately stored in cooling boxes with ice packs. The refrigerated samples were taken to the Life Sciences laboratories of the Salesiana Polytechnic University (Girón-Quito). The transit time was 1 to 3 hours. The market samples were analyzed within 24 hours or immediately upon reaching the laboratory.

Figure 1. Location of markets in the Metropolitan District of Quito-Ecuador. S An, San Antonio; Pom, Pomasqui; Cal, Calderón; Cara, Carapungo; Cot, Cotocollao; Ken, La Kennedy; Ame, La América; S Cl, Santa Clara; Iña, Iñaquito; Cen, Centro; Qui, Quinche; L Gr, Llano Grande; Com, Comité del Pueblo; Pue, Puembo; Yar, Yaruquí; Cum, Cumbayá; Tum, Tumbaco; S Ro, San Roque; Mag, La Magdalena; S Ba, San Bartolo; Sol, Solanda; Chi, Chiriacu; May, Mayorista; Ecu, La Ecuatoriana; El Ca, El Canal; Ci Ib, Ciudadela Ibarra; Gua, Guamaní; Con, Conocoto; Ala, Alangasí; Ama, Amaguaña
Measurements Performed on Foods
The bromatological analyses performed on the fresh watercress (Nasturtium officinale R. Br.), lettuce (Lactuca sativa L.), lemon balm (Melissa officinalis L.) and taraxacum (Taraxacum officinale (L.) Weber ex F.H. Wigg) were size (equatorial and longitudinal diameter in cm, weight in g, humidity in %, ash in %, soluble solids in °Brix, titrable acidity in % and pH. The soluble solids were quantified as described by INEN-ISO-2172 13, with a Hand-Refractometer RHC-200ATC (Huake, China), while a S40 SevenMulti pHmeter (Mettler Toledo, Belgium) was used to measure out the pH. Titulable acidity and relative density were determined as described by INEN-ISO-750 14 and INEN-391 15. A humidifier HB4 3-S Halogen (Mettler Toledo, Belgium) was used to measure the humidity and dry material, while the ash was measured as described by 16.
Heavy Metal Analysis
Sample preparation
The samples were dried at 70 ºC for 24 hours in a KBF 240 air recirculation oven (Binder, Germany). The dried samples were ground in a primary IKA 11 mill, stored in a glass bottle and hermetically sealed. The sample was treated as described by AOAC-999.10 17, with modifications. Approximately 250 mg of the pulverized sample was mixed in a Teflon digester with 1.5 mL of concentrated nitric acid and 1.5 mL of sulfuric acid. The mixture was digested in a Speedwave HB43-S Digestion (Berghof, Germany) for 40 min according to the specifications of the equipment manual. The digested mixture was graduated in 25 mL with deionized water.
Atomic absorption
The calibration curve was built on the same day with concentrations of lead and cadmium standards separately from 0.5, 1.0, 2.0 and 4.0 ppm. Quantification was performed on a SpectrAA-55 Atomic Absorption Spectrophotometer (Varian-Agilent Technologies, United States), as described by AOAC-999.10 17. The lead was quantified at 217.0 nm and the Cadmium at 228.8 nm. All the digested mixture was read three times, and the concentration was expressed in mg/kg dry weight (DW).
Microbiological and Parasitological Analysis
The most widely accepted and used techniques are those recommended by WHO for a total count of microorganisms in plant materials. According to the methodology of the WHO, 10 g of fresh sample was suspended in 90 mL of sodium chloride-peptone buffer at a pH of 7.0. A fresh sample was plated in duplicate to count total aerobic bacteria and incubated at 30-35 ºC for 48 h 18.
For the parasitological analysis, dilutions 10-1 were made with the plant extract and sterile water, centrifuged at 3000 rpm for 5 min, and the pellet formed was removed and placed in an Eppendorf tube. After this, a slide with 0.2 µL of sample and 0.2 µL of violet crystal was placed in an optical microscope, and the presence of parasites or cysts was observed.
Statistical analysis
Statistical differences were determined by analysis of variance (simple ANOVA). The mean separation was made via a Tukeyʼs test with 0.01 significant differences, and correlations by Pearson with a 99 % confidence level were employed to estimate the possible significance of the samples. Moreover, Principal Components Analysis (PCA) was applied to select the variables most influencing the differences between samples. The INFOSTAT 2009 software was used for statistical analyses.
RESULTS
In Tables 1 to 4 found Physicochemical properties, microbiological results, and heavy metals.
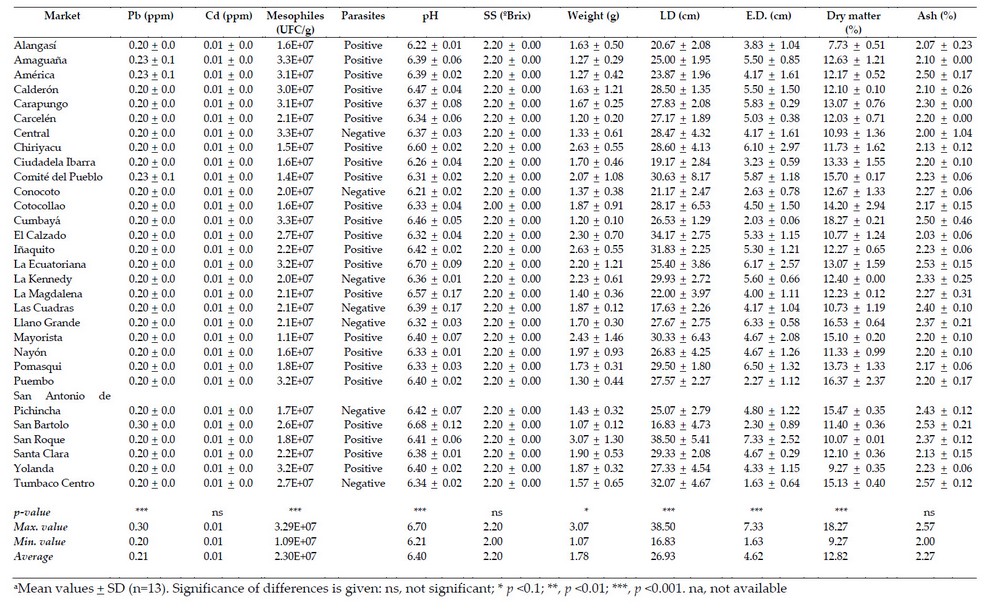
Table 1. Average value (FW) of parameters related to the commercial quality of taraxacum (T. officinale) a

Table 2. Average value (FW) of parameters related to the commercial quality of lemon balm (M. officinale) a
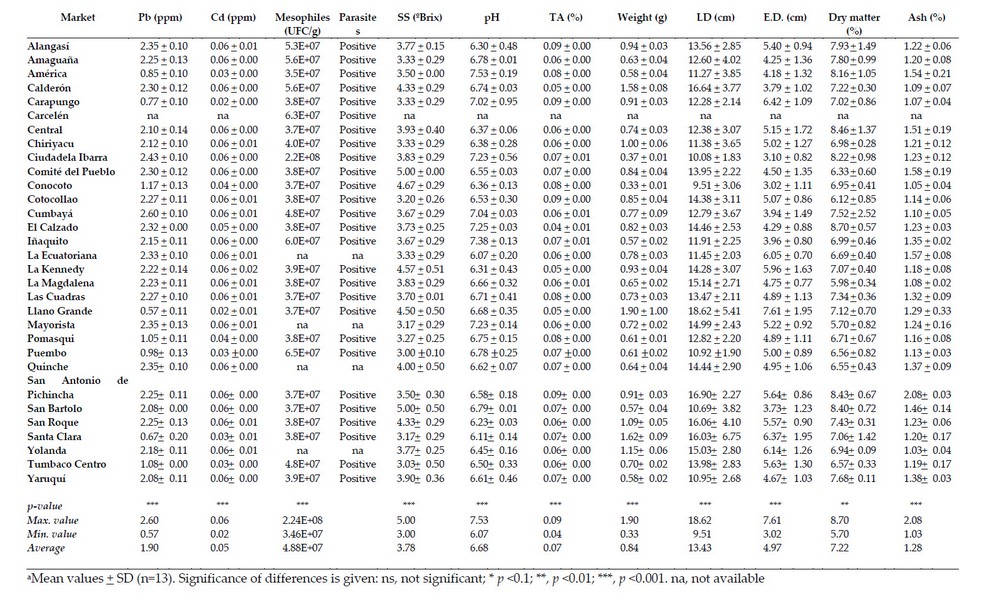
Table 3. Average value (FW) of parameters related to the commercial quality of watercress (N. officinale) a
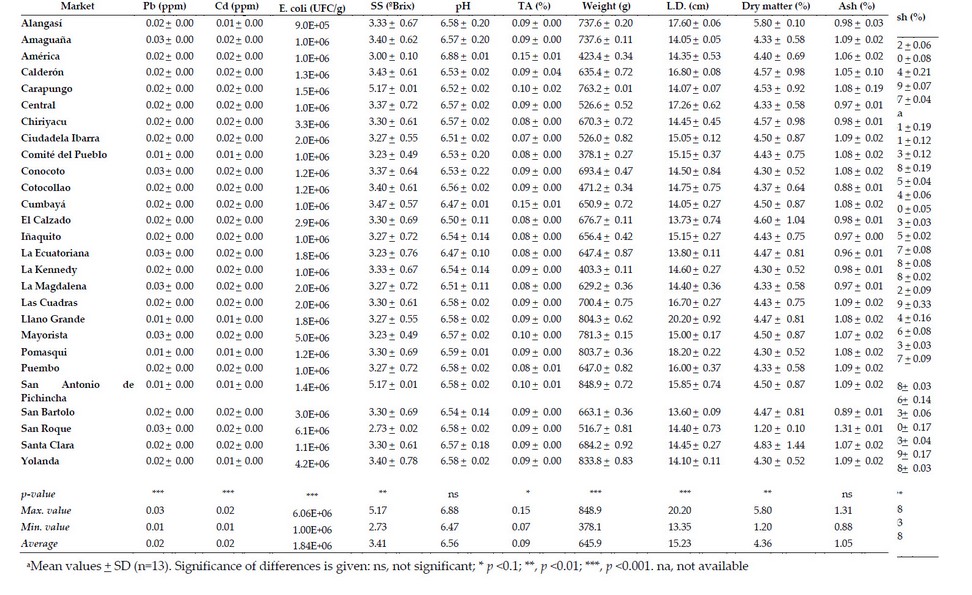
Table. Average value (FW) of parameters related to the commercial quality of lettuce (L. sativa) a 4
DISCUSSION
Estimation of food quality
Size
The selected markets correspond to 55.5 % of the total in the Metropolitan District of Quito. The markets that presented the highest values of equatorial and longitudinal diameter were Santa Clara and Llano Grande for lemon balm, San Roque for taraxaco and Llano Grande for lettuce and watercress (Table 1 to 4). Thus, in most cases, the markets located to the north of the city showed enormous sizes; this may be because these markets are provided with products from the Pichincha province, while the markets of the center and south are supplied with products from the Cotopaxi province, marking the difference in said products. The average longitudinal diameter of lemon balm was 5.3 cm, less than reported by 19, which showed values of longitudinal diameter of 9 cm and equatorial diameter of 7 cm. On the other hand, the statistical differences shown by the analyzed samples of lemon balm indicate that at least one market has different diameters than the others, agreeing with that reported by other authors that suggest that it is sold in the markets regardless of the size of the leaf, causing maximum use of the therapeutic properties presented by the species the other hand, the longitudinal diameter (13.43 cm) and the equatorial diameter (4.97 cm) of the watercress showed values similar to those reported by other authors who indicated a length of 15 to 20 cm.
Weight
The markets that presented the highest weights of the samples under study were San Roque for taraxaco, San Bartolo for lemon balm, Llano Grande for watercress and San Antonio de Pichincha for lettuce (Table 1 to 4). On the other hand, the markets that presented the highest dry matter values were Cumbayá for taraxaco, Carcelén for lemon balm, El Calzado for watercress and Alangasí for lettuce. About the previous data, the weight values showed a relationship with the data obtained from dry matter. Thus, the markets located to the south of the city presented higher values of humidity and low weights; this again could be due to the place of origin of the samples since most of the products that reach the wholesale market located south of the city can be from the province of Cotopaxi.
The distribution of dry matter was lemon balm (14.3 %), taraxacum (12.8 %), watercress (7.2 %) and lettuce (5.8 %). The dry matter values of the watercress were similar to those reported by other authors, which showed 94.6 % humidity 20,21.
The markets with the highest ash values were Tumbaco Centro for Taraxaco, La Ecuatoriana and Solanda for lemon balm, San Antonio de Pichincha for watercress and San Roque for lettuce. Thus, in most cases for taraxaco and lemon balm, the ash values exceeded that established by the Ecuadorian Technical Norm of ash of 2 % 22. This was also observed in the simple of cress obtained from San Antonio de Pichincha, who reported values of 2.08 %. The ash values of lemon balm, taraxaco and watercress showed significant differences in the place of sales, while the lettuce did not present differences.
Soluble solid (SS.)
The markets that presented higher values of SS were the Comité del Pueblo and San Bartolo for watercress, Carapungo and San Antonio de Pichincha for lettuce (Table 1 to 4). In addition, the average values for SS classified from highest to lowest for the samples under study were watercress (3.78 ºBrix), lettuce (3.41 ºBrix), taraxaco and lemon balm (2.20 ºBrix). All samples analyzed except taraxaco and lemon balm did not show significant differences when considering all the markets under study.
On the other hand, the markets that presented the highest pH values were La Ecuatoriana for taraxaco, Iñaquito for lemon balm, and America for watercress and lettuce. In addition, the average values from highest to lowest for pH were lemon balm (6.83), watercress (6.68), lettuce (6.56), taraxaco (6.40). These values are similar to those reported by other authors 7. Concerning the statistical analysis of the pH, considering all the markets in the study, these showed significant differences, except the lettuce, which did not differ.
Heavy metal analysis
Lead
The markets with the highest values of Pb were San Bartolo for taraxaco, Alangasí for lemon balm, Cumbayá for watercress and several markets in the case of lettuce (Table 1 to 4). These data indicate that no specific region accumulates heavy metals; this may be due to the fact that the soils of the Sierra region are volcanic and have high amounts of heavy metals.
The taraxaco, lemon and lettuce balm showed average values of 0.02 ppm in most cases and 1.90 ppm for watercress. Thus, at least one market showed statistical differences concerning the values reported for the remaining markets under study. The concentrations of Pb for lemon balm, taraxaco and lettuce are within the limits accepted by the Ecuadorian Technical Standard that reports maximum values of 0.5 ppm 22 and 0.3 ppm established by CODEX and the European Union Regulations for heavy metals 23. However, the values of watercress exceed the maximum limits, becoming a health risk since lead can replace calcium accumulating in bone tissues, as suggested by other authors 24.
Although taraxaco is considered a bioindicator of environmental contamination, as suggested by other authors 25, it has a low concentration compared to the rest of the samples under study.
Cadmium
Lemon balm and taraxaco showed a Cd value of 0.01 ppm for the studied markets. Therefore, all the needs under study did not show significant differences in the values reported. In the case of watercress and lettuce, these showed concentrations of 0.05 and 0.02 ppm, respectively, in most of the different markets under study, and these values corresponded to the maximum concentration. Cadmium, in general, should be absent in plant tissues; however, its presence may be related to the type of soil in which it is grown, which corresponds to the inter-Andean region whose areas show the presence of heavy metals of a geographical nature from the Cordillera of the Andes, as suggested by other authors 26. On the other hand, according to the limits established by the FAO (4.2 ppm), the CODEX and the European Union Regulation for heavy metals 23 with reference limits of 0.2 ppm, the results obtained for the samples under study did not exceed the permissible limit.
PCA analysis for food quality
Applied to our data set, PCA revealed that the first principal components explain 29.6 % of lettuce, 33.1 % of lemon balm, 37.06 % of watercress, and 44.0 % of taraxaco of total variance. A plot of the scores of the first factor (PC1) versus the second principal component (PC2), i.e., the projection of the samples along the directions identified by the first two PCs, is reported in Figure 2. PC1 was mainly linked to lead in most of the samples under study, all with positive loading values in this component.

Figure 2. Scores plot of the watercress (N. officinale) (A), lemon balm (M. officinalis) (B), lettuce (L. sativa) (C) and taraxaco (T. officinale) (D) using the two first principal components obtained by PCA of weight, dry material, ash, lead, Cadmium, pH, soluble solids and titulable acidity.
Microbiological quality
Mesophilic Aerobic Microorganisms
The average of mesophilic aerobic microorganisms in all samples exceeded the values of 4 log10 CFU/g (1×104 CFU/g (Table 1 to 4). The results of lettuce were similar to the study of 2, who reported ranged from a geometric mean of 3.50 to 8.39 log CFU/g in lettuce, parsley and radish; 27 found values of >6 log CFU/g in environmentally friendly romaine lettuce, and 28 presented ranges between 5 to >7 log10 CFU/g for organic vegetables and 3 to >7 log10 CFU/g for conventional vegetables, including ʽLooseleafʼ, ʽButterheadʼ, ʽRomaineʼ and ʽRed looseleafʼ lettuce.
The high load of microorganisms could be due to the contamination between the transports of vegetables from the farm to the market. This possible contamination was also reported by 29 who observed that the counts of indicator microorganisms increased from farm to market, for example, mean Enterobacteriaceae and Listeria spp. Counts were on average higher by 0.9 log CFU/g and by 0.5 log CFU/g respectively at market compared to farm, indicating that between farm and market, vegetables are either contaminated or that there are conditions that allow the growth of microorganisms.
On the other hand, the pH values for taraxaco, lemon balm, watercress and lettuce were 6.4, 6.8, 6.7 and 6.5, respectively. The pH values found could explain the high microbial quantity in the different species, as indicated 30, who points out that the bacteria count may be low when pH is low (presence of acidic substances). However, at neutral or higher pH (5 to 8.5), the level of contamination of the herbal preparations may be higher.
Parasites
The present study evaluated the presence or absence of eggs and cysts of parasites in the different species. Thus, the watercress and lettuce showed the highest identification of parasites eggs or cysts, followed by lemon balm and taraxaco (Table 1 to 4). Some authors suggest that the watercress structure favors the level of contamination due to the multiple and separated leaves, which allows a greater adherence of parasites 31. In addition, this type of plant is developed in highly humid soil and mainly inaccessible areas that probably carry contaminated water, as suggested 32. On the other hand, the lettuce has broad leaves, firmly juxtaposed, which hinders the adhesion of protozoan cysts, eggs and helminth larvae; another hypothesis is that many helminth eggs can survive for more extended periods in the environment, which could justify the higher frequencies of parasites found in the watercress, whose cultivation requires land permanently humid, as proposed others authors 32. Also, fascioliasis risk is linked with the consumption of raw vegetables such as lettuce, suggesting contamination when washing vegetables with untreated water and in-plant cultures using natural water for irrigation, as indicated 33.
PCA analysis for microbiological quality
The number of microorganisms for taraxacum and lemon balm according to PCA statistical analyses (Figure 3) is influenced highly by pH, with values of 0.56 and 0.42 for the first principal component, respectively, what means that at basic or neutral pH value, the growth of aerobic mesophilic microorganisms is more significant than an acid pH values. Also, the percentage of ash influences the number of mesophilic aerobic organisms in the case of taraxaco, lettuce and watercress, with values of 0.64 and 0.28 for the first principal component, respectively.
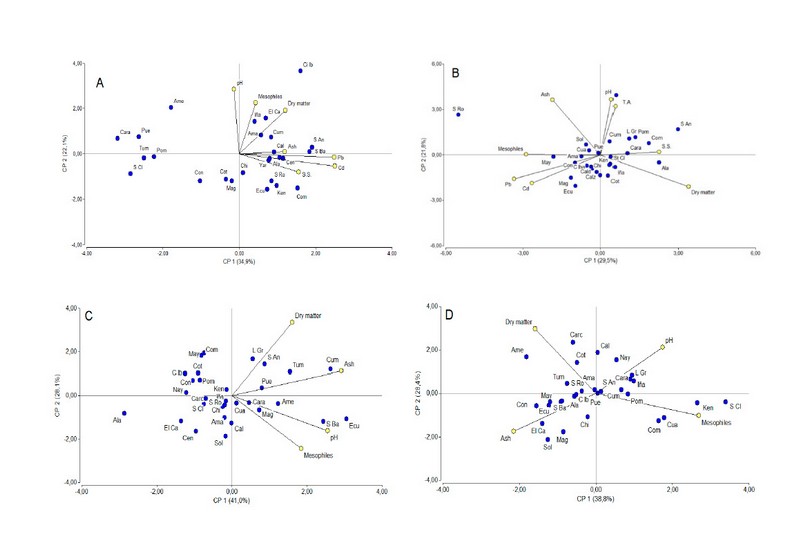
Figure 3. Scores plot of the watercress (N. officinale) (A), lettuce (L. sativa) (B), taraxacum (T. officinale) (C) and lemon balm (M. Officinalis) (D) using the two first principal components obtained by PCA of weight, dry material, ash, pH, soluble solids, titrable acidity, mesophiles and parasites
CONCLUSIONS
The present study supports the idea that lemon balm, taraxaco, watercress and lettuce meet the bromatological, microbiological and heavy metals requirements in the Distrito Metropolitano de Quito markets. In general, the size of the products under study showed better characteristics for needs in the north and center of the city. At the same time, heavy metals did not exceed the limits allowed by Ecuadorian regulations such as CODEX and European regulations (0.3 ppm for Pb and 0.2 ppm for Cd).
On the other hand, according to our microbiological results, it is suggested that the species studied have lower quality hygiene (outside acceptable microbiological limits). The study results show that 76 to 100% of the samples commercialized in markets from Quito were contaminated with at least one parasite structure on overage. Therefore, improvement is needed in sanitary conditions during the planting and distribution of vegetables to reduce the contamination and the risks to food safety to humans. Vegetables likely to be eaten raw should not be obtained from areas where these practices are known, or suspected, to be unhygienic.
Author Contributions: methodology, Coyago Elena, Méndez Gabriela; formal analysis, Acurio Daniel.; investigation, Coyago Elena.; resources, Coyago Elena.; writing—original draft preparation, Méndez Gabriela, Coyago Elena, Acurio Daniel; writing—review and editing, Coyago Elena, Acurio Daniel, Méndez Gabriela; project administration, Coyago Elena.; funding acquisition, Coyago Elena. All authors have read and agreed to the published version of the manuscript.
Funding: «This research received no external funding.»
Conflicts of Interest: «The authors declare no conflict of interest.»
REFERENCES
1. Garrido ML, Veitia SA, Guillen TDA, García OC, Chacón JJ. Procedimiento analítico para la determinación de metales pesados en zanahoria y espinaca cultivadas en organopónicos urbanos. Rev Ciencias Técnicas Agropecu. 2013;22(1):20–6.
2. Faour-Klingbeil D, Murtada M, Kuri V, Todd E. Understanding the routes of contamination of ready-to-eat vegetables in the Middle East. Food Control [Internet]. 2016;62:125–33. Available from: http://dx.doi.org/10.1016/j.foodcont.2015.10.024
3. Santiago J, Atsumi C, Garcia F, Da-Silveira A, Paiao S, Carrion A, et al. Parasitological analysis of green leaf lettuce cultivated in different production systems. Semin Agrar Londrina. 2017;38(2):801–8.
4. Cerna-Cortes JF, Leon-Montes N, Cortes-Cueto AL, Salas-Rangel LP, Helguera-Repetto AC, Lopez-Hernandez D, et al. Microbiological quality of ready-to-eat vegetables collected in Mexico City: Occurrence of aerobic-mesophilic bacteria, fecal coliforms, and potentially pathogenic nontuberculous mycobacteria. Biomed Res Int. 2015 Mar;2015:789508.
5. Carrillo L, Audisio C, Bejarano N, Gómez S. Manual de microbiologia de alimentos. Aliment en España. 2001;125–32.
6. Coyago-Cruz E, Méndez G, Acurio D, Valdés L, Quishpe K, González C, et al. Microbiological and heavy metal risk in alfalfa juice (Medicago sativa) sold in markets. Ital J Food Sci. 2018;(June):25–9.
7. Vílchez H. Evaluación económica de berros (Nasturtium officinale) hidropónicos a través del estudio de casos de productores de la región metropolitana. Chile: Universidad de Talca (Chile). Escuela de Agronomia; 2008.
8. Mendiola M, Montalbán J. Plantas aromáticas gastronómicas. España: Ediciones Paraningo S.A.; 2009.
9. Guamán R. Estudio bioagronómico de 10 cultivares de lechuga de cabeza (Lactuca sativa), utilizando dos tipos de fertilizantes orgánicos, en el cantón Riobamba, Provincia de Chimborazo. 2010.
10. Vélez Bravo AP, Ortega González JE. Determinación de coliformes totales y E. Coli en muestras de lechuga expendidas en cuatro mercados de la ciudad de Cuenca [Internet]. Cuenca; 2013. Available from: http://dspace.ucuenca.edu.ec/handle/123456789/4301
11. Chen J, Wei F, Zheng C, Wu Y, Adriano DC. Background concentrations of elements in soils of China. Water Air Soil Pollut. 1991;57–58(1):699–712.
12. INEN1750. NTE INEN 1750:1994. Hortalizas y frutas frescas. Muestreo. Inst Ecuatoriano Norm. 2012;
13. INEN2172. NTE INEN-ISO 2172. Jugo de Frutas-Determinación del contenido de sólidos solubles- método picnométrico (ISO 2172:1983, IDT). Inst Ecuatoriano Norm. 2014;1–5.
14. INEN750. NTE-INEN-ISO 750:2013 Productos vegetales y de frutas – Determinación de la acidez titulable (IDT). Inst Ecuatoriano Norm. 2013;1998:1–5.
15. INEN391. Norma Técnica Ecuatoriana NTE INEN 391 : 2012 Segunda revisión. Conservas vegetales. Jugos de frutas. Determinación de la densidad relativa. Inst Ecuatoriano Norm. 2012;1–6.
16. Harbers L. Ash analysis, in Food analysis. Aspen Publ. Indiana; 1998.
17. AOAC999.10. AOAC Official Method 999.10 Lead, Cadmium, Zinc, Cooper, and Iron in Foods. Atomic absorption spectrophotometry after microwave digestion. Off methods Anal AOAC Int. 2010;17–9.
18. WHO. Quality control methods for herbal materials. Malta: WHO Library Cataloging-in-publication Data; 1998.
19. Tucker A, DeBaggio T. The encyclopedia of herbs. A comprehensive reference to herbs of flavor and fragrance. DeBaggio F, editor. London: Timber Press, Inc.; 2009.
20. Patiño J, Ocampo J. Diseño y construcción de un equipo reductor de tamaño de berro, espinaca, zanahoria. Chimborazo; 2014.
21. INCAP, OPS. Tabla de composición de alimentos de Centroamérica. Segunda. INCAP, editor. Panamá; 2012. 128 p.
22. INEN2392. NTE INEN 2392. Hierbas aromaticas (requisitos). Serv Ecuatoriano Norm. 2017;4:3.
23. Codex-Alimentarius. Informe de la 22a reunión del Comite del CODEX sobre aditivos alimentarios y contaminantes de los alimentos. La Haya, Países Bajos 19-24 de marzo de 1990. Programa Conjunto FAO/OMS Sobre Normas Aliment Com del Codex Aliment [Internet]. 1991;1(1):2–5. Available from: http://thesis.ekt.gr/thesisBookReader/id/1834#page/104/mode/2up
24. Mahmood Q, Rashid A, Ahmad SS, Azim MR, Bilal M. Current status of toxic metals addition to environment and its consequences. In: Anjum N, Ahmad I, Pereira E, Duarte A, Umar S, Khan N, editors. The Plant Family Brassicaceae. 21st ed. Springer, Dordrecht; 2012. p. 35–69.
25. Kolář J, Seňková J. Reduction of mineral nutrient availability accelerates flowering of Arabidopsis thaliana. J Plant Physiol. 2008;165(15):1601–9.
26. Pozo W, Sanfeliu T, Carrera G. Metales pesados en humedales de arroz en la cuenca baja del río Guayas. Maskana. 2011;2(1):17–30.
27. Ryu JH, Kim M, Kim EG, Beuchat LR, Kim H. Comparison of the Microbiological Quality of Environmentally Friendly and Conventionally Grown Vegetables Sold at Retail Markets in Korea. J Food Sci. 2014;79(9):M1739–44.
28. Maffei DF, Silveira NF de A, Catanozi M da PLM. Microbiological quality of organic and conventional vegetables sold in Brazil. Food Control [Internet]. 2013;29(1):226–30. Available from: http://dx.doi.org/10.1016/j.foodcont.2012.06.013
29. Ssemanda JN, Reij M, Bagabe MC, Muvunyi CM, Joosten H, Zwietering MH. Indicator microorganisms in fresh vegetables from «farm to fork» in Rwanda. Food Control [Internet]. 2017;75:126–33. Available from: http://dx.doi.org/10.1016/j.foodcont.2016.12.031
30. De-Freitas M, Bauab T. Microbial quality of medicinal plants materials. In: Isin A, editor. Latest research into Quality Control. Published. Croatia; 2010. p. 67–82.
31. Célia L, Lessa T, Ramos P, Grassi R, Bombonato P, Ambrósio C. Arquitetura da arvore bronquica no sagui-de-tufo: Um modelo animal experimental para lesoes do sistema respiratório. Arch Vet Sci. 2012;17(4):63–9.
32. Soares B, Cantos GA. Detecção de estruturas parasitárias em hortaliças comercializadas na cidade de Florianópolis, SC, Brasil. Rev Bras Ciencias Farm J Pharm Sci. 2006;42(3):455–60.
33. Zumaquero-Ríos J, Sarracent-Pérez J, Rojas-García R, Rojas-Rivero L, Martínez-Tovilla Y, Valero M, et al. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: Epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis. 2013;7(11).
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation: Coyago-Cruz E, Méndez G , Acurio D. Assessment of the microbiological quality and heavy metal risk in vegetable species marketed in outdoor fairs. Revis Bionatura 2023;8 (4) 18. http://dx.doi.org/10.21931/RB/2023.08.03.18




















