Vol 8 No 2 2023 – 27

Epidemiological Study of Prevalence TB in Iraq
Asmaa A. AL-Kaisse*1, Amina N.AL-Thwani1, Ahmed A. Mankhi2, Zainab H. Abood1 and Ruqaya Mustafa Ali3
1 Institute of Genetic Engineering and Biotechnology for Post Graduate Studies/ Baghdad University; email: asmaa.adnanalqaisi@yahoo.com
2 National Specialized Center for Chest and Respiratory Diseases, Ministry of Health and Environment, Baghdad /Iraq.
3 Ministry of Agriculture, Veterinary Department /Baghdad / Iraq.
* Correspondence: mailto: asmaa.adnanalqaisi@yahoo.com
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.27
ABSTRACT
To assess the prevalence of tuberculosis epidemic in Iraq in terms of the age groups most affected by tuberculosis bacteria, for both gender and for all governorates of Iraq, various clinical specimens were obtained from 744 patients attending the Specialized Chest and Respiratory Disease Center / National Reference Laboratory (NRL) for tuberculosis in Baghdad between April 15 and November 14 2021 the diagnosis by direct microscopy using the Zeihl-Nelsen (ZN) stain and followed by culturing on Lowenstein-Jensen medium (LJ), for 744 clinical specimens revealed that 92(12.37%) specimens were positive by direct examination while 111(14.9%) specimens were positive by culturing on LJ medium with sputum specimens accounting for the majority of culture positive specimens 103/111(92.8%) the rate of Pulmonary tuberculosis (PTB) was a higher than (EPTB) extra-pulmonary (94.6%), (5.4%) respectively the most of tuberculosis cases were found in Baghdad city (62.2%) vs other governorates (37.8%) males were more affected (63.0%) than females (37.0%) and the majority of patients were aged 35–44 years (30.6%) but the lowest age group was least than 15 years (1.8%) the data above ** (P≤0.01) showed a statistically significant difference, cultivation dependence most be more sensitive than direct method and require more attention in TB control programs to healing patients.
Keywords: Tuberculosis; Mycobacterium tuberculosis; Iraq.
INTRODUCTION
Infectious tuberculosis, or TB, is a leading cause of death and poor health worldwide; anyone can contract it. Before the coronavirus (COVID-19) pandemic, TB was the most significant cause of death from a single infectious disease. Higher than HIV/AIDS in terms of both treatability and preventability is tuberculosis (TB). A six-month pharmacological regimen effectively treats about 85% of people with TB illness 1. TB infection can be treated with a regimen lasting one to six months. 1.3 million more people will die from tuberculosis-related causes in 2020 than from HIV/AIDS-related causes combined (0.68 million). The COVID-19 outbreak has impacted TB mortality more than HIV/AIDS in 2020. The number of people who died from HIV/AIDS fell between 2019 and 2020 2, in contrast to those who died from tuberculosis. Most droplets released from the mouth, nose, and throat when someone talks, or coughs transmit tuberculosis, a respiratory disease. However, tuberculosis can also spread to other body regions, such as the lymph nodes, pleura, belly and urogenital tract 3, 4, skin and joints 5, 6, bones 7, 8, and the meninges. Extra-pulmonary tuberculosis is the name given to the following type of illness. Although no signs or symptoms of tuberculosis may be present, a continuing immune response to Mycobacterium tuberculosis (M. tb) antigens is what the World Health Organization (WHO) considers an LTBI. The World Health Organization estimates that approximately one-fourth of the world’s population is infected by Mtb and has a latent TB infection (LTBI) 4. To reduce the risk of poor treatment outcomes, health sequelae, as well as the negative social and economic consequences of tuberculosis, the first goal is to ensure that tuberculosis disease is exposed early and treated promptly; the second goal is to reduce community-level TB disease prevalence, thus preventing the spread of tuberculosis in the future in fetuses and children. There is a connection between these two objectives. Preventative tuberculosis (TB) treatment can be provided to individuals diagnosed with the disease, decreasing the chance of future cases of illness 5. TB and other kinds of tuberculosis are affected by this (TB). According to WHO research, the frequency of tuberculosis in Iraq will reach 27 cases per 100,000 people by the year 2020, according to WHO. A steady decline in disease prevalence had occurred throughout the prior decade 6. The goal of this research was to find out how common tuberculosis is among Iraqi patients.
MATERIALS AND METHODS
Collection of Samples To investigate Mycobacterium tuberculosis, a total of 744 diagnostic specimens were collected from patients who went to the “Specialized Chest and Respiratory Diseases Center/National Reference Laboratory for Tuberculosis (NRL) in Baghdad” between April 15 and November 14 2021. There were 429 males, which accounts for 60.0 percent of the total, and 315 females, which accounts for 37.0 percent. The ages of the individuals ranged from a few months to over 65 years. The specimens were divided into two categories: those with pulmonary tuberculosis 603 and those with extra-pulmonary tuberculosis 141 (Table 1).
Sample Processing: To digest and disinfect the specimens, standard operating protocols for laboratory manual labor were followed 7. Ziehl Neelsen (ZN) stain was employed in smear microscopy, which is a form of direct inspection. This was done to examine all clinically decontaminated specimens.
Cultivation: Each decontaminated specimen was injected into LJ media by spreading three to four drops of centrifuged sediment all over the surface of three slops of LJ medium. This was done in order to culture the bacteria. After having each culture sit at an angle for three days at 37 degrees Celsius with the lids only partially closed, the lids were secured, and the cultures were incubated vertically for a total of eight weeks (cultures were examined weekly). After this incubation period, the growth was either observed or dismissed as negative, and the results were recorded according to whether they were positive or negative. It takes anywhere from six to eight weeks for the consequences of solid culture to become visible. The growth rate, colony morphology, and ZN staining of positive cultured isolates were also used to gain further identifications.
RESULTS
Colony morphology and staining
During 8 weeks, the results of culture on LJ media became seen. M. tuberculosis complex colonies on LJ media were big, uneven in form, scratchy, inflexible, and non-pigmented, similar to coli flower colonies. Bacilli were straight or slightly curved red-dotted rods organized singly or in pairs when microscopic culture isolates were inspected with ZN dye.
Coverage of the database
According to the results obtained, pulmonary TB (PTB) specimens accounted for 603(81%) of the total 744 specimens, whereas extra-pulmonary TB (EPTB) specimens accounted for 141(19%), as indicated in Table (2). Smear microscopy (direct examination) revealed that 92(12.37%) specimens were positive, 111(14.9 %) specimens were able to develop on LJ media, and culture examination revealed 633 negative specimens. ** (P≤0.01), a very significant difference between all category specimens. The positive cultivation rate of M. tuberculosis complex from pulmonary tuberculosis (PTB) specimens was more excellent 105/111(94.6%) than extra-pulmonary tuberculosis (EPTB) specimens 6/111(5.4%), with a highly significant difference ** (P≤ 0.01) between them. Sputum specimens 103/111(92.8%) had the highest proportion of bacterial growth out of 111 specimens, whereas PTB specimens 103/105 had a higher rate (98.1%).
Tuberculosis prevalence in Baghdad and neighboring governorates
Table (3) shows that Bagdad City had a more significant percentage of culture-positive specimens than other governorates, with 69/111(62.2%) specimens being positive by cultivation on LJ media, compared to 42/111 (37.8%) in other governorates, with very significant dereference ** (P< 0.01), shows.
Connection of Tuberculosis with Gender and Age
The data presented in Table (4) indicated that males had a significantly higher prevalence of tuberculosis than females; the overall male-to-female attribution was 1.7 (70/41), and the variation was statistically significant. The data also indicated that the ratio of males to females was significantly different (P<0.01). The patients were placed in one of the Table’s seven distinct age groups, which were assigned to them following their ages in Table (5). The age groups (35–44 years) exhibited higher frequencies of TB cases (30.6 percent) than any other age group. In comparison, the age groups (15 years) with a percentage (1.8 percent) were revealed to be the least recorded age groups, with highly significant differences between all age groups ** (P< 0.01).

Table 1. Specimen numbers and kinds

Table 2. Division of specimens according to the kind of TB and test.

Table 3. Apportionment of tuberculosis cases in Baghdad and other governorates

Table 4. Divide TB patients according to gender
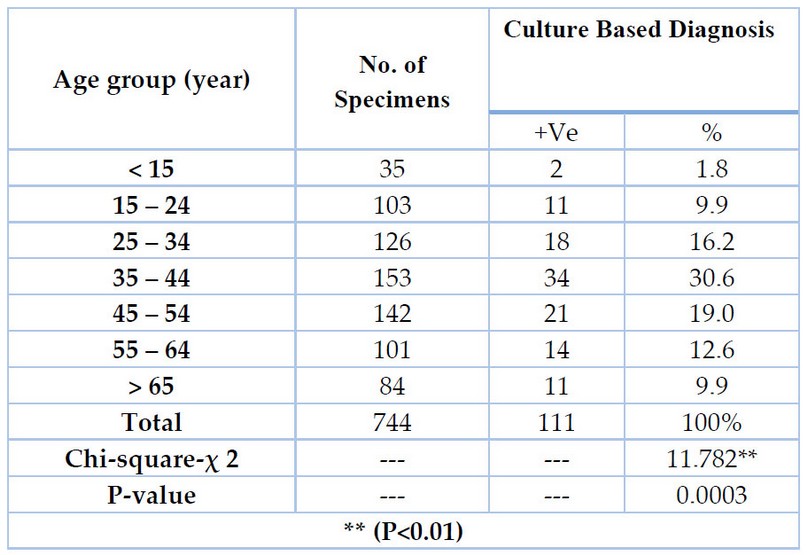
Table 5. Patients diagnosed with tuberculosis are classified according to age group.
DISCUSSION
The direct smear microscopy method applied frequently in the third world and particularly in Iraq, is one of the methods that can be used to diagnose tuberculosis. Regarding the diagnosis of tuberculosis, the LJ culture medium is usually recognized as the gold standard in underdeveloped nations 8. In terms of the clinical manifestations that they cause, pulmonary tuberculosis (also known as PTB) and extra-pulmonary tuberculosis (also known as EPTB) are the two most frequent types of tuberculosis (TB). The pleura, lymph nodes, bones, and the meningeal lining of the brain are not the only organs that tuberculosis (TB) can affect; they can also affect other organs (extra-pulmonary tuberculosis, EPTB). Even though pulmonary tuberculosis is the most common form, it is not the only kind of tuberculosis (PTB).
Pulmonary TB (PTB) expands at a quick rate in 94.6 percent of cases, with the remaining incidences of the disease happening outside of the lungs in 5.4 percent of patients (EPTB). This investigation determined that PTB’s prevalence was higher than EPTB’s, aligning with earlier studies’ findings 9,10,11,12. According to a study that was conducted in Iraqi Kurdistan 13, it was found that 63.5% of patients had extra-pulmonary tuberculosis, whereas only 36.5% of patients had pulmonary tuberculosis. Extra-pulmonary tuberculosis is a condition that affects a significant portion of people who have tuberculosis. There are several potential explanations for why the risk of extra-pulmonary tuberculosis varies from person to person; several of these features include the inherent immunity of the host, the pathogenicity of various strains of Mycobacterium tuberculosis, and the mechanism of transmission. According to the findings of yet another Iranian study 14,15, patients diagnosed with extra-pulmonary tuberculosis face a mortality risk of 5.58 times higher than those diagnosed with pulmonary tuberculosis.
On the other hand, the sputum smear test can differentiate between two types of pulmonary tuberculosis, which are referred to as smear-positive and smear-negative pulmonary tuberculosis, respectively. In this experiment, 92.8 percent of the total sputum samples showed positive results for pulmonary tuberculosis on a smear test; the fact that it poses a danger of infecting others by droplet or airborne transmission 16,17 is one reason why it could be considered a source of community contagiousness. In addition, the findings demonstrated that patients with EPTB were more likely to have pleural infections than any other type of infection, which is consistent with 9,18 in Iraq and 19,20 in Iran and Uzbekistan; further research has found that lymph nodes are to blame for nearly half of the cases of extra-pulmonary tuberculosis (EPTB) in Iraqi Kurdistan 21 and in Turkey 22. For instance, the genitourinary system and the skin were the two most common sites of infection in Hong Kong 23, but in the United States, bone and joint infections were more prevalent 24.
In this investigation, the M. tuberculosis cultivation ratio in clinical specimens was 14.9%, significantly higher than the percentage that could be determined with a detailed visual examination (12.37 percent ). Smear microscopy and Ziehl–Neelsen (ZN) staining are the two methods most commonly used to diagnose tuberculosis in underdeveloped countries (TB). This strategy does not require the utilization of advanced technology 25, among its many appealing features, low expense, great performance, and a high degree of precision. It takes two hours to receive the results of the smear microscopy, but it is less sensitive than other testing procedures since it requires between 5000 and 10,000 bacteria in a milliliter of sputum to achieve a positive result. Consequently, it takes longer to get the results of the smear microscopy. Patients with a positive culture but a negative sputum smear account for 13% of all cases of tuberculosis that are spread from patient to patient. When healthy persons come into close contact with TB suspects with negative sputum cultures, the risk of getting MTB and developing active TB increases for both sets of individuals. The test’s sensitivity can be improved by following a routine in which sputum samples are collected on three separate days first thing in the morning. As a result of its lack of sensitivity, sputum smear microscopy cannot differentiate between Mycobacterium tuberculosis and Mycobacterium tuberculosis complex 26.
Consequently, the Lowenstein–Jensen (LJ) medium for the growth of Mycobacterium is utilized in tuberculosis diagnosis. This medium is recognized as the gold standard. This technique has high sensitivity, even though it can take many weeks to complete. Cultures of Mycobacterium TB grown in LJ medium can detect the presence of Mycobacterium tuberculosis in sputum samples when there are at least 10 viable bacilli per milliliter of sample 27. Regardless of the incidence of HIV, sputum induction detected approximately 75% of culture-positive TB cases in children and adults, according to a meta-analysis that included 17 independent research. However, the percentages estimated by individual investigations varied 28. Researchers came to this conclusion after investigating the sensitivity of the sputum induction process. The generation of sputum was typically successful, with success rates ranging from 76.4 percent to 100 percent, and side effects were uncommon and modest, according to a new comprehensive examination of 23 trials. This review came to the additional conclusion that the majority of adverse effects were controllable. Compared to culture, the range of sensitivity that can be achieved by using microscopy on induced sputum samples is 0% to 100%. When compared to nasopharyngeal aspiration and stomach lavage, sputum induction produced significantly larger yields 29.
Only three extra-pulmonary specimens were found to have ZN stain, whereas six solid culture specimens were found to contain it. This difference is likely because ZN stain is only present in extra-pulmonary models. Even though pulmonary tuberculosis is the most common form of the disease in children, it is rarely tested for because children have trouble coughing up enough phlegm to get a good specimen for bacteriological verification. This is even though pulmonary tuberculosis is the most common disease in children. In children, the risk of contracting pulmonary tuberculosis is significantly higher than other kinds of tuberculosis. In some instances, it has been reported that the organism spreads through the bloodstream. This has been observed most frequently in infants and children under the age of five. The disease, which is often referred to as «military tuberculosis,» can be spread through traumatic means and can affect any organ or tissue in the body, including the bones, brain, meninges, and stomach. The most prevalent indications of extra-pulmonary tuberculosis in children are TB adenitis and TB pleural effusion. The extra-pulmonary form of tuberculosis affects approximately 20–30% of all children who get the disease. If children with tuberculosis (TB) are not given the appropriate medication or develop a resistant strain, treatment may become substantially more challenging for these patients 30. The AFB smear is quite specific for Mycobacterium; nonetheless, it is essential to remember that a positive AFB result might be caused by any non-tuberculous mycobacteria (NTM).
The acid resistance of Nocardia and other actinomycetes is not as high as that of other actinomycetes, although these species are uncommon. In a positive AFB smear, mycobacteria are almost invariably present; however, M. tuberculosis is not a prerequisite for mycobacteria 31. Mycobacterium culture is, as of the time of this writing, the method that is both the most sensitive and trustworthy in terms of determining whether or not a current case of tuberculosis exists 32,31,33. If the smear test returns negative results for tuberculosis, a residual culture must be performed. DNA fingerprinting and DST are two methods that can be utilized to identify culture isolates; these culture isolates can subsequently be used in molecular epidemiology research, culture can be performed on any sample, and sputum is the most usually used material for diagnosing pulmonary tuberculosis 33, but culture can be performed on any sample also Mycobacteria can be found at a density of 10–100 viable cells per milliliter of material when culture procedures are used 34. Because of the limited sensitivity of the direct smear method, live bacteria may be missed while using this method 35. There is a higher probability of infection when acid-resistant bacteria are not tested for beforehand.
It is essential to detect pulmonary tuberculosis as early as possible and make an accurate diagnosis to cut down on the disease’s transmission rate and the severity of its long-term effects. The sputum smear microscopy test is the most common test used to determine whether a patient has lung tuberculosis. This test is widely available in a variety of countries around the world. According to a recent study 36,37,38, most tuberculosis patients with a positive smear test have high-quality microscopy of two consecutive sputum specimens. This percentage ranges from 95 to 98 percent. This finding was uncovered by scientists working in the United States. The World Health Organization (WHO) advises that only two sputum specimens should be collected on the same day, and this recommendation can only be obtained through high-grade acid-fast bacilli (AFB) microscopy. Consequently, diagnostic costs can be brought down to a more manageable level by reducing the number of patients who withdraw from the procedure 38.
The more significant number of tuberculosis cases in Baghdad may be attributable to the city’s high population density and crowded conditions (close contact with patients), as well as the availability of more diagnostic methods facilities than in other governorates, which allowed for the recording of more tuberculosis cases among suspected TB patients and patients who were unable to travel to Baghdad and who went to the TB center in their governorate. According to the data presented in Table 3, Baghdad has a significantly greater rate of TB patients than the national average. Because it is the only accredited laboratory in Iraq to perform such tests, patients with suspected tuberculosis bring their samples to the National Reference Laboratory (NRL) for identification and culture. This is done because the NRL is the only place in Iraq where such tests can be performed. The high incidence of tuberculosis in Baghdad can be attributed to several different factors. The most important of them are the growing population, the increasing urbanization, and the presence of people in enclosed areas for the majority of the day; all of these factors are contributing to an increase in the number of refugees and displaced persons living there. Because HIV, smoking, diabetes, alcoholism, and malnutrition are all key risk factors and social determinants of tuberculosis (TB), the significance of these factors has come under increased scrutiny over the past several years 39; the list is rounded out by overcrowding, the challenge of successfully navigating day-to-day life, and a lack of resources. Tuberculosis is the most significant cause of mortality and disability in the developing world and the primary source of HIV infection in the world’s poorest countries, according to the World Health Organization (WHO) 40,41.
The findings presented in Table (4) align with those found in studies 42 and research 43,9,44, which indicate that men risk developing tuberculosis three times higher than women. According to the findings of a study that was carried out in Iran, males have a higher risk of developing pulmonary tuberculosis than females 45. Around the world, the proportion of males to females among newly diagnosed tuberculosis (TB) cases with a positive smear test is approximately two to one. This is not the case, however, in the country of Pakistan. The rate of tuberculosis cases reported by females is 20–30 percent higher than those reported by males, and this difference remains even with increasing age 46. As a point of interest, the disparity in mortality rates between men and women persists throughout all stages of life. Those who are HIV-positive and have a history of tuberculosis have a greater chance of passing away from the disease than those who are HIV-positive and have an account of tuberculosis but are male. Even more so for women in Africa, where several studies have discovered a fatality rate that is 20% higher for HIV/TB co-infected women than for co-infected men 47. This is especially true for women in Africa. In nations like South Africa, the rate of HIV-associated tuberculosis fatalities among women with HIV and tuberculosis is also twenty percent greater. It is more common for males to develop tuberculosis than women because men partake in more activities outside the home, such as smoking, consuming alcohol, and engaging in behaviors that are against the law. Among the many different examples, one that has a significant connection to these connections is bottomless pit mining 48.
Comparable to this, the fact that women have connections with tuberculosis patients who are not members of their immediate family or household can be considered a risk factor 49. Pregnant women with tuberculosis and HIV infection may have an increased chance of dying from tuberculosis (TB) because of the medical problems that suppress the immune system during pregnancy 50. Because of these chromosomal and hormonal variances, the host’s response to an infection differs in males and females. This is because the immune system is regulated differently. Estradiol seems to be the hormone responsible for maintaining a healthy immune system, in contrast to progesterone and testosterone, which appear to inhibit the body’s natural defenses against infectious diseases. Genetic variables, such as those connected with a person’s sex chromosomes, can also play a role in determining an individual’s vulnerability to infection 51. Research has shown that characteristics related to a person’s line of work and their way of life play a significant part in the risk of contracting an illness and its treatment. When researching tuberculosis, it is customarily a good idea to focus on those who fall into high-risk categories, such as those who are incarcerated; vaccinations against tuberculosis, on the other hand, offer protection to children against the illness 52.
As seen in Table (5), the incidence of tuberculosis in children under 15 is relatively low, which may be attributed to the widespread use of the BCG vaccine beginning in childhood. The effectiveness of immunization is highest in children but declines with increasing age 53. These occurrences are far more likely to occur during puberty and the early years of young adulthood. There is a marked decrease in cases among children and adolescents between the ages of 10 and 14. For these evaluations, the most extensive tuberculosis surveillance dataset that is currently available was utilized: 54. This dataset includes data from a diverse range of countries, many of which have a high endemicity of tuberculosis, as well as a range of epidemiological and topographical characteristics that are indicative of many different regions across the globe. According to the results of this survey, an overwhelming majority of respondents possess complete knowledge of tuberculosis transmission. Accurate information regarding the spread of tuberculosis is associated with various other factors. These characteristics include being between the ages of 35 and 44, having completed secondary or higher education, coming from a wealthy family, being exposed to all three types of mass media, working in a professional, technical, or managerial capacity, and residing in an urban area. Other characteristics include being born into a family with a high level of education and being exposed to all three types of mass media. The study’s findings indicated a significant connection between the self-reported age of individuals in Malawi who received accurate information regarding the spread of tuberculosis and their actual age 55. Those older than 25, in particular, better understood how tuberculosis was spread. Adults have a higher risk of contracting tuberculosis (TB) than children do. In our most recent study, we found that those between the ages of 25 and 34 had a positivity rate of 16.2 percent, those between the ages of 35 and 44 had a positivity rate of 30.6 percent, and those 55 and older had a positivity rate of 19.0 percent. Although tuberculosis is most frequently found in adult males, people of any age can become infected with the disease 56. According to a report published by the World Health Organization, the prevalence of tuberculosis in Iraq has been steadily declining across various age groups 6.
CONCLUSIONS
Our results suggest that cultivation dependence is more sensitive than the direct method. On the other hand, require more attention in TB control programs to help patients prevent infection and healing.
Funding: Self-funded this research.
Informed Consent Statement: Statement Regarding Informed Consent The Specialized Chest and Respiratory Disease Center / National Reference Laboratory (NRL) in Baghdad has given their announced written agreement for studying tuberculosis.
Acknowledgments: We are pleased to extend our thanks to all the medical staff at “Specialized Chest and Respiratory Disease Center / National Reference Laboratory (NRL) for Tuberculosis” in Baghdad
Conflicts of Interest: None
REFERENCES
1. World Health Organization. Global Tuberculosis Report 2021. https://doi.org/10.1016/j.ijid.2021.02.107
2. World Health Organization . TB Data [website]. Geneva.2021: (https://worldhealthorg.shinyapps.io/tb_pronto/).
3. Sharma, S.K., Mohan, A. and Kohli, M. Extra-pulmonary tuberculosis. Expert Review of Respiratory Medicine, 2021. 15(7), pp.931-948. https://doi.org/10.1080/17476348.2021.1927718
4. Khabibullina, N.F., Kutuzova, D.M., Burmistrova, I.A. and Lyadova, I.V. The biological and clinical aspects of a latent tuberculosis infection. Tropical Medicine and Infectious Disease, 7(3), p.48.Tuberculosis Infection. Trop. Med. Infect. Dis., 2022. 7, 48. https://doi.org/10.3390/tropicalmed7030048
5. World Health Organization,. WHO consolidated guidelines on tuberculosis: module 2: screening: systematic screening for tuberculosis disease. 2021. World Health Organization. https://archive.lstmed.ac.uk/id/eprint/17349
6. World Health Organization. 2020. Global Tuberculosis Report. Incidence of Ttuberculosis (Per 100,000 People).
7. Ahmed, M. M. Tuberculosis Situation in Iraq: A puzzle of Estimates. In. J. Mycobacter, 2013. 2(4): 248-249. https://doi.org/10.1016/j.ijmyco.2013.10.002
8. Shoukrie, A., Alameen, A., Shaban, D., Alamari, M., Aboguttaia, N. and Askar, N. The Yield of Sputum Smear Direct Microscopy Using Ziehl-Neelsen Stain in Comparison with Lowenstein Jensen Culture on the Diagnosis of Pulmonary Tuberculosis in Tripoli-Libya. Mycobact Dis, 2018. 8(1). DOI: 10.4172/2161-1068.1000256.
9. Ahmed, S.T., Ali, R.M. and Shihab, B.A. Prevalence of tuberculosis infection among Iraqi patients. World Journal of Pharmaceutical Research, 2018. 7(1), pp.1383-1394. DOI: 10.20959/wjpr20181-10557.
10. Ali, S.M., Al-Faham, M.A. and Mankhi, A.A. Identification and Discrimination of Mycobacterium tuberculosis Complex with Traditional and Real-Time PCR in Different Specimens in Iraq. Journal of the Faculty of Medicine Baghdad, 2020. 62(3). https://doi.org/10.32007/jfacmedbagdad.6231787 .
11. Alateah, S.M., Othman, M.W., Ahmed, M., Al Amro, M.S., Al Sherbini, N. and Ajlan, H.H. A retrospective study of tuberculosis prevalence amongst patients attending a tertiary hospital in Riyadh, Saudi Arabia. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases, 2020. 21, p.100185. https://doi.org/10.1016/j.jctube.2020.100185
12. Eddabra, R. and Neffa, M. Epidemiological profile among pulmonary and extra-pulmonary tuberculosis patients in Laayoune, Morocco. The Pan African Medical Journal, 2020. 37. https://doi.org/10.11604%2Fpamj.2020.37.56.21111
13. Balaky, S.T.J., Mawlood, A.H. and Shabila, N.P. Survival analysis of patients with tuberculosis in Erbil, Iraqi Kurdistan region. BMC Infectious Diseases, 2019. 19(1), pp.1-8. https://doi.org/10.1186/s12879-019-4544-8
14. García-Rodríguez, J.F., Álvarez-Díaz, H., Lorenzo-García, M.V., Mariño-Callejo, A., Fernández-Rial, Á. and Sesma-Sánchez, P. Extrapulmonary tuberculosis: epidemiology and risk factors. Enfermedades infecciosas y microbiologia clinica, 2011. 29(7), pp.502-509. https://doi.org/10.1016/j.eimc.2011.03.005
15. Rahmanian, V., Rahmanian, K., Rahmanian, N., Rastgoofard, M.A. and Mansoorian, E. Survival rate among tuberculosis patients identified in south of Iran, 2005-2016. Journal of Acute Disease, 2018. 7(5), p.207. DOI: 10.4103/2221-6189.244172
16. WHO. Global Tuberculosis Report World Health Organization: Geneva, Switzerland. Available Online: 2020. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (Accessed on May 5 2020).
17. WHO. Tuberculosis; World Health Organization: Geneva, Switzerland. Available online: 2020 https://www.who.int/news-room/fact-sheets/detail/tuberculosis (Accessed on May 17 2020).
18. M Jumaah, H. The roles of radiology and ESR in the diagnosis of tuberculosis in young military males in Iraq. Annals of the College of Medicine, Mosul, 2013. 39(2), pp.182-185. http://dx.doi.org/10.33899/mmed.2013.81270
19. Nikonajad, A., Azimi, S.A., Allami, A., Qasemi Bargi, R. and Tabarraei, A. Epidemiology of extrapulmonary tuberculosis in Northeast of Iran. Medical Laboratory Journal, 2021. 15(1), pp.1-7. http://dx.doi.org/10.29252/mlj.15.1.1
20. Abdugapparov, F., Grigoryan, R., Parpieva, N., Massavirov, S., Riskiyev, A., Gadoev, J., Buziashvili, M., Tukvadze, N., Hovhannesyan, A. and Dadu, A. Diagnostic Procedures, Diagnoses, and Treatment Outcomes of Patients with Presumptive Tuberculosis Pleural Effusion in Uzbekistan. International Journal of Environmental Research and Public Health, 2021.18(11), p.5769. https://www.mdpi.com/1660-4601/18/11/5769#
21. Merza, M.A. A 5-year experience characterizing the demographic and clinical profile and directly observed treatment short-course treatment outcome in National Tuberculosis Center of Duhok province, Iraqi Kurdistan. SAGE Open Medicine, 2020. 8, p.2050312120921055. https://doi.org/10.1177%2F2050312120921055
22. Aygün D, Akçakaya N, Çokuğraş H, Camcıoğlu H. Clinical Manifestations and Diagnosis of Extra-pulmonary Tuberculosis in Children. J Pediatr Inf; 2019. 13(2):e74-e79. DOI: 10.5578/ced.201922
23. Noertjojo, K., Tam C, M., Chan S, L. and Chan-Yeung MM, W. Extra-pulmonary and pulmonary tuberculosis in Hong Kong. The International Journal of Tuberculosis and Lung Disease, 2002. 6(10), pp.879-886. 879-886. PMID: 12365574.
24. Yang, Z., Kong, Y., Wilson, F., Foxman, B., Fowler, A.H., Marrs, C.F., Cave, MD and Bates, J.H. Identification of risk factors for extra-pulmonary tuberculosis. Clinical infectious diseases, 2004. 38(2), pp.199-205. https://doi.org/10.1086/380644
25. Riaz, M., Mahmood, Z., Javed, M.T., Javed, I., Shahid, M., Abbas, M. and Ehtisham-ul-Haque, S. Drug resistant strains of Mycobacterium tuberculosis identified through PCR-RFLP from patients of Central Punjab, Pakistan. International journal of immunopathology and pharmacology, 2016. 29(3), pp.443-449. https://doi.org/10.1177%2F0394632016638100
26. Caulfield, A.J. and Wengenack, N.L. Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases, 2016. 4, pp.33-43. https://doi.org/10.1016/j.jctube.2016.05.005
27. Munir, M.K., Rehman, S., Aasim, M., Iqbal, R. and Saeed, S. Comparison of Ziehl Neelsen microscopy with GeneXpert for detection of Mycobacterium tuberculosis. IOSR J Dent Med Sci, 2015. 14(11), pp.56-60. DOI: 10.9790/0853-1411105660
28. Gonzalez-Angulo, Y., Wiysonge, C.S., Geldenhuys, H., Hanekom, W., Mahomed, H., Hussey, G. and Hatherill, M. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. European journal of clinical microbiology & infectious diseases, 2012. 31(7), pp.1619-1630. https://doi.org/10.1007/s10096-011-1485-6
29. Hepple, P., Ford, N. and McNerney, R. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. The international journal of tuberculosis and lung disease, 2012. 16(5), pp.579-588. https://doi.org/10.5588/ijtld.11.0617
30. WHO. Global Tuberculosis Report. 2018. Vol. 69, Pharmacological Reports. 2017. 683-690 p.
31. Association of Public Health Laboratories. Mycobacterium Tuberculosis: Assessing Your Laboratory. Silver Spring, 2009.MD: APHL.
32. American Thoracic Society and the Centers for Disease Control. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This Official Statement of the American Thoracic Society and the Centers for Disease Control and Prevention was Adopted by the ATS Board of Directors, July 1999. This Statement was Endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med; 2000.161(4 Pt 1):1376-95. https://doi.org/10.1164/ajrccm.161.4.16141
33. American Thoracic Society.Diagnostic Standards and Classification of Tuberculosis. Am Rev Respir Dis; 1990. 142(3):725-35. https://doi.org/10.1164/ajrccm/142.3.725
34. El Khechine, A., Henry, M., Raoult, D. and Drancourt, M. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology, 2009. 155(7), pp.2384-2389. https://doi.org/10.1099/mic.0.026484-0
35. Ambreen, A., Jamil, M. and Mustafa, T. Viable Mycobacterium tuberculosis in sputum after pulmonary tuberculosis cure. BMC infectious diseases, 2019. 19(1), pp.1-8. https://doi.org/10.1186/s12879-019-4561-7
36. Davis, J.L., Cattamanchi, A., Cuevas, L.E., Hopewell, P.C. and Steingart, K.R. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. The Lancet infectious diseases, 2013. 13(2), pp.147-154. https://doi.org/10.1016/S1473-3099(12)70232-3
37. Ryu YJ . Diagnosis of Pulmonary Tuberculosis: Recent Advances and Diagnostic Algorithms. Tuberc Respir Dis (Seoul); 2015. 78(2):64–71. https://doi.org/10.4046/trd.2015.78.2.64
38. World Health Organization. Same-Day Diagnosis of Tuberculosis by Microscopy: WHO policy Statement. Geneva: World Health Organization; Available from: https://apps.who.int/iris/bitstream/handle/10665/44603/9789241501606_eng. pdf; jsessionid=613A37FA332657028E190531EB53D770? sequence=1. [Accessed January 25 2019] 2011. https://doi.org/10.1016/j.ijid.2019.03.021
39. Creswell, J., Raviglione, M., Ottmani, S., Migliori, G.B., Uplekar, M., Blanc, L., Sotgiu, G. and Lönnroth, K. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. European Respiratory Journal, 2011. 37(5), pp.1269-1282. DOI: 10.1183/09031936.00084310.
40. Duarte, R., Gomes, M., Oliveira, A., Sousa, P., Franco, I. and Gaio, A.R. Social profile of the highest tuberculosis incidence areas in Portugal. Revista Portuguesa de Pneumología, 2016. 22(1), pp.50-52. https://doi.org/10.1016/j.rppnen.2015.08.006
41. Sousa, P., Oliveira, A., Gomes, M., Gaio, A.R. and Duarte, R. Longitudinal clustering of tuberculosis incidence and predictors for the time profiles: the impact of HIV. The International Journal of Tuberculosis and Lung Disease, 2016.20(8), pp.1027-1032. https://doi.org/10.5588/ijtld.15.0522
42. Amiri, H., Mohammadi, M.J., Alavi, S.M., Salmanzadeh, S., Hematnia, F., Azar, M. and Rahmatiasl, H. Capture-recapture based study on the completeness of smear positive pulmonary tuberculosis reporting in southwest Iran during 2016. BMC Public Health, 2021.21(1), pp.1-10. https://doi.org/10.1186/s12889-021-12398-w
43. Ali, Z.A., Al-Obaidi, M.J., Sameer, F.O., Mankhi, A.A., Misha’al, K.I., Jassim, IA, Taqi, EA and Ad’hiah, A.H. Epidemiological profile of tuberculosis in Iraq during 2011–2018. Indian Journal of Tuberculosis, 2022. 69(1), pp.27-34. https://doi.org/10.1016/j.ijtb.2021.01.003
44. Hamasaeed, P.A. Microscopic and Molecular Diagnosis of Mycobacterium Tuberculosis in Erbil city-Iraq. Journal of University of Babylon for Pure and Applied Sciences, 2019. 27(4), pp.20-29. https://www.journalofbabylon.com/index.php/JUBPAS/article/view/2298
45. Nazar, E., Baghishani, H., Doosti, H., Ghavami, V., Aryan, E., Nasehi, M., Sharafi, S., Esmaily, H. and Yazdani Charati, J. Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018. International Journal of Environmental Research and Public Health, 2021. 18(1), p.54. https://doi.org/10.3390/ijerph18010054
46. Codlin, A.J., Khowaja, S., Chen, Z., Rahbar, M.H., Qadeer, E., Ara, I., McCormick, J.B., Fisher-Hoch, S.P. and Khan, A.J. Gender differences in tuberculosis notification in Pakistan. The American journal of tropical medicine and hygiene, 2011. 85(3), p.514. https://doi.org/10.4269%2Fajtmh.2011.10-0701
47. Getahun, H., Sculier, D., Sismanidis, C., Grzemska, M. and Raviglione, M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. Journal of Infectious Diseases, 2012. 205(suppl_2), pp.S216-S227. https://doi.org/10.1093/infdis/jis009
48. Z. Al-Fayyadh, D. .; Hasson, A. A. .; Hussein, A. K. .; Hassan, R. K. EFFECT OF HUMIC ACID SPRAY ON GROWTH CHARACTERISTICS OF WHEAT VARIETIES. JLSAR 2020, 1, 10-19.
49. Crampin, A.C., Glynn, J.R., Floyd, S., Malema, S.S., Mwinuka, V.K., Ngwira, B.M.M., Mwaungulu, F.D., Warndorff, D.K. and Fine, PEM Tuberculosis and gender: exploring the patterns in a case control study in Malawi. The international journal of tuberculosis and lung disease, 2004. 8(2), pp.194-203.
50. Noaman A I, Khalaf R M, Emad GH, Al-Abbasy, Mohammed Th. T. Effect of flaxseed oil dosing on fertility, growth characteristics and some physical, biochemical, and hormonal blood parameters during the early pregnancy of Awassi ewes. Revis Bionatura. 2022;7(4) 5. http://dx.doi.org/10.21931/RB/2022.07.04.5.
51. Laetitia Gay, Cle´ a Melenotte, Ines Lakbar, Soraya Mezouar, Christian Devaux, Didier Raoult, Marc-Karim Bendiane, Marc Leone, and Jean-Louis Mège. Sexual Dimorphism and Gender in Infectious Diseases. Frontiers in Immunology | 2021.| Volume 12 | Article 698121. https://doi.org/10.3389/fimmu.2021.698121
52. Schito, M., Migliori, G.B., Fletcher, H.A., McNerney, R., Centis, R., D’Ambrosio, L., Bates, M., Kibiki, G., Kapata, N., Corrah, T. and Bomanji, J. Perspectives on advances in tuberculosis diagnostics, drugs, and vaccines. Clinical infectious diseases, 2015. 61(suppl_3), pp.S102-S118. https://doi.org/10.1093/cid/civ609
53. Neyrolles, O. and Quintana-Murci, L. Sexual inequality in tuberculosis. PLoS medicine, 2009. 6(12), p.e1000199. https://doi.org/10.1371/journal.pmed.1000199
54. World Health Organization. Global tuberculosis database. www.who.int/tb/publications/global_report/en/. Date last accessed: 2017 November 13, 2017.
55. Ou, Y., Luo, Z., Mou, J., Ming, H., Wang, X., Yan, S. and Tan, A. Knowledge and determinants regarding tuberculosis among medical students in Hunan, China: a cross-sectional study. BMC Public Health, 2018. 18(1), pp.1-7. https://doi.org/10.1186/s12889-018-5636-x
56. Gaur, P.S., Bhaskar, R., Singh, S., Saxena, P. and Agnihotri, S. Incidence and clinical profiles of pulmonary and extra-pulmonary tuberculosis patients in North Indian population: a hospital based retrospective study. International Journal of Research and Development in Pharmacy & Life Sciences, 2017. 6(5), pp.2773-2778. https://doi.org/10.21276/IJRDPL.2278-0238.2017.6(5).2773-2778
Received: 10 February 2023/ Accepted: 15 May 2023 / Published:15 June 2023
Citation: AL-Kaisse A A, AL-Thwani A N, Mankhi A A. Abood Z H, Ali R M. Epidemiological Study of Prevalence TB in Iraq. Revis Bionatura 2023;8 (2) 27. http://dx.doi.org/10.21931/RB/2023.08.02.27



















