Vol 7 No 4 2022- 61

Antibiogram of Eucalyptus and Sesame seed oil against clinical isolates of Pseudomonas aeruginosa
1,2Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq
*Corresponding author: E-mail: rasha.jk.bio@gmail.com,
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.61
ABSTRACT
Because of the frequent use of antibiotics, which leads to the occurrence of resistance by pathogenic bacteria, and for the benefit of oils and the ease of their availability with minor side effects, thus, the study aimed to investigate the anti-microbial activity of Sesame seed oil and Eucalyptus against P. aeruginosa bacteria. Ten clinical isolates of Pseudomonas aeruginosa were previously isolated from wounds and have multi-drug resistance to common antibiotics. The results of Sesame oil against the bacterial isolates showed no antibacterial activity not only in the agar well diffusion method but also in the disc diffusion method. At the same time, the findings showed that eucalyptus oil had an antibacterial activity with concentrations (20%,40%, 60%, 80%, and 100%) against these isolates. Further, the antibiotics susceptibility test results showed that bacterial isolates were resistant to Amikacin, sensitive to each of cefotaxime, chloramphenicol and levofloxacin, and the effect of Gentamicin differed against the tested bacteria. Our finding concludes that eucalyptus oil may be used as an alternative drug in the treatment as an external ointment for wound infection by Pseudomonas aeruginosa.
Keywords: Antibiotic-resistant, disc diffusion method, pathogenic bacteria, the wounds, plant essential oils
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen that causes disease and death in patients with cystic fibrosis and those with impaired immune systems. P. aeruginosa is a Gram-negative aerobic bacterium. It can thrive in temperatures ranging from 4 to 42 degrees Celsius and survives with low nutrition levels 1. These properties enable it to cling to and live on medical equipment and other hospital surfaces, making infections more likely in immunocompromised individuals 2. Patients with cystic fibrosis are at a higher risk of morbidity and mortality due to the chronic infections that P. aeruginosa can produce. These infections can lead to lung damage, deficiency, pneumonia, and urinary tract infections. P. aeruginosa can also cause bacteremia 3,4. P. aeruginosa infections are notoriously difficult to cure because the bacteria possess a high level of innate resistance and can develop resistance to a wide range of medications. Metallo-b-lactamases (MBL), altered penicillin protein binders (PBP), porin mutations, plasmid enzymatic modification, DNA-gyrase mutation, and active expulsion pumps are all examples of broad-spectrum b-lactamases. These are just some of the many mechanisms that P. aeruginosa uses to evade antibiotics 5. Carbapenemic drugs (imipenem and meropenem) are broad-spectrum antibiotics used to treat P. aeruginosa nosocomial infections. The formation of metalloenzymes, a lack of porin permeability and a rise in the expression of active expulsion pumps are all linked to Carbapenemic resistance 6. The development of MBL is linked to carbapenem-resistant P. aeruginosa, which can, except for aztreonam, hydrolyze all b-lactam antibiotics 5,7. Because of the active substances found in various components, plants have drawn researchers from all over the world as a source of treatment. For decades, plant oils and extracts have been used for several purposes. In today’s world, antibiotic resistance in microorganisms is a grave concern 8. As a result, herbal remedies derived from plants are considered safe alternatives to synthetic drugs. The antibacterial activity of extracts and plant oils, in particular, paved the way for various applications, including food preservation, medicines, and cosmetics. Therapies and alternative medicine plant oils are aromatic oily fluids obtained through steam distillation from different plant parts like flowers, shoots, seeds, grasses, stems, leaves, forests, fruits, and stocks. Compared to antibiotics, these oils have been shown to be highly effective antibacterial agents. These have anti-microbial activities against viruses, bacteria, and fungi, including antibacterial, antifungal, anticancer, antiviral, and anti-oxidant characteristics 9. The genus of Eucalyptus is an aromatic and medicinal plant that is widely distributed around the world. It has therapeutic properties because it produces bioactive chemicals interacting with other species in the environment, preventing bacterial and fungal growth 10, 11. Candidates for creating novel anti-microbial medications include compounds that can suppress infections while causing little harm to host cells. Today, plant-based treatments are regaining favor because the efficacy of antibiotics, which are widely regarded as almost universal solutions for infectious diseases, is waning. This is due to the widespread use of these chemical agents and their prescription on a large scale, which is sometimes inappropriate, resulting in bacterial strain adaptability and selection strains that are multiresistant and pose a public health risk 12. Eucalyptus is a native Australian genus in the Myrtaceae family, with approximately 900 varieties and subspecies. Eucalyptus contains bioactive chemicals. These chemicals have been shown to have antibacterial, antifungal, anti-inflammatory, analgesic, and anti-oxidant properties 10, 13. Sesame, also known as Sesamum indicum L., is a crop that has been farmed for a very long time and has antifungal characteristics. It is believed that it originated in the continent of Africa. The anti-oxidant and health-promoting effects of sesame oil, along with a rise in the rate of fatty acid oxidation in the mitochondria and peroxisomes of the liver, can be attributed to sesame oil 14. Sesame oil consumption appears to increase plasma gamma-tocopherol and vitamin E activity, which are thought to aid in cancer and heart disease prevention., as well as antibacterial activity 15.The widespread use of antibiotics causes pathogenic bacteria to develop resistance, and because of the convenience with which oils may be obtained with the fewest side effects. Thus, the study aimed to examine if sesame seed oil and Eucalyptus oil could limit the growth of P. aeruginosa.
MATERIALS AND METHODS
Bacterial isolates
All isolates of P. aeruginosa previously isolated from patients from the deep skin surface of the wound using sterile swabs, then cultured directly on blood agar and MacConkey agar aerobically overnight at 37 c for bacteria and identified by Vitek 2 system, kept in refrigerator till beginning the work.
Extraction of volatile oil from Eucalyptus and fixed oil of Sesame
Leaves of Eucalyptus that were collected from the University of Baghdad, Aljadiryaa campus, after cleaning and rinsing leaves were dried and ground finely and placed in the Clevenger apparatus to extract the volatile oil using hydro distillation method by applying a ratio 1:5 between plant and distilled water (16). Meanwhile, the sesame seed is ground to a paste and heated to 80-90 c for 15 min. Then add enough boiling water to suspend the ground seed on stirring for 15 min. The upper oil layer separated after cooling and dried by heating 17. Sesame seed oil was obtained from the local market, but its origin is Iran; after we sell it, kept at 4℃ until use.
Antibacterial activity of extracted oil
A- Agar well diffusion method
In the first step, activation of bacterial isolates in (BHI) broth and incubate the tubes at 37°C for 18-24hr. Making dilute the bacterial broth in normal saline compared with Mcfarland 0.5 tube (1×108 CFU/ml). Then, culturing on Mueller Hinton agar (MHA) plates were prepared sterilely. Inoculation (MHA) plates with the diluted tested bacteria by the Swabbing method. We added 200µl from each sesame oil concentration (100000µg/ml,50000µg/ml,25000µg/ml), while Eucalyptus oil concentrations (20%, 40%, 60%, 80%,100%) were put in three wells and one well full by (DMSO) used as control( since we dissolve the oil with it) and incubated the plates at 37°C for (18-24hr). Finally, detected inhibition zone for anti-microbial activity.
B- Agar disc diffusion method:
Using the activation of bacterial isolates previously, then diluted the bacterial broth and cultured on (MHA) as mentioned above. Adding 0.2ml of each sesame seed oil concentration (5000 µg/ml,10000 µg/m,25000 µg/ml,50000 µg/ml) was used to the submerged disc that we synthesized it using sterile filter papers. In contrast, the control disc was soaked with DMSO and put on the surface of a cultured (MHA) plate aseptically and incubated at 37 for (18-24hr) to detect the inhibition zone as a clear zone around the discs and measured in mm using a ruler.
Antibiotic susceptibility test for P. aeruginosa
The Kirby-Baurer method was used to test anti-microbial sensitivity. The antibiotics that were used in this study were Amikacin, Azithromycin, cefotaxime, chloramphenicol, Gentamicin, and levofloxacin. The isolated colonies were collected using a sterile loop and emulsified in 3-4 ml of sterile physiological saline. A sterile brush that had been dampened in the bacterial suspension was then used to make streaks across the surface of the Mueller Hinton agar. The anti-microbial disc was diced using antiseptic forceps, and then it was regularly distributed on the inoculated plate. After incubating the plate aerobically at 37°C for 24 hours, the zone of growth inhibition around each disc was measured in millimeters, and the results were reported as sensitive, intermediate, or resistant to a specific anti-microbial agent by comparison with standard inhibition zones as mentioned in the clinical laboratories standards institute. 6.
Statistical Analysis
The SPSS, IBM version 20, program was used to perform the analysis on the data18. Statistical significance is assumed when the p-values are less than 0.05.
RESULTS
Antibiotics sensitivity test for Pseudomonas aeruginosa
The antibiotic sensitivity test results showed that the isolates of P. aeruginosa were resistant to Amikacin with a diameter of inhibition zone 14 mm and 12 mm, respectively. Azithromycin differs in its effect against the two test isolates, 8mm and 19mm, respectively. The two isolates of P. aeruginosa were sensitive to each of the cefotaxime, chloramphenicol, and levofloxacin antibiotics, while the effect of Gentamicin differed against the two tested isolates. Isolates 1 to 6 were sensitive to Gentamicin with a diameter of inhibition zone 16mm, while isolates 7 to 10 were resistant to the same antibiotics with a diameter of inhibition zone 12 mm (figure 1).

Figure 1. Antibiotics susceptibility test for P. aeruginosa
Antibacterial activity of sesame seed oil against P. aeruginosa
The results of the antibacterial activity of sesame seed oil against P. aeruginosa by the two methods are shown in Figures 2 and 3. The bacterium P. aeruginosa offers high resistance to the concentrations used in both agar well methods and disc diffusion of sesame seed oil, which is unable to inhibit their growth.

Figure 2. Resistance pattern of P. aeruginosa against sesame oil in agar well diffusion method.
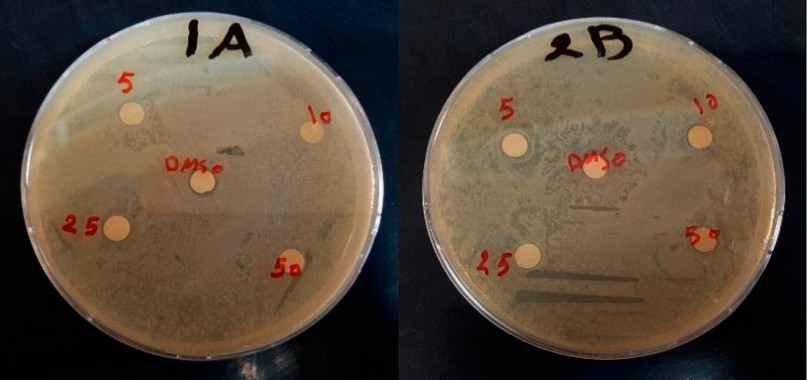
Figure 3. The effect of sesame oil on P. aeruginosa in the agar disc diffusion method.
Antibacterial activity of Eucalyptus oil against P. aeruginosa
Figure 4 shows the different concentrations of Eucalyptus oil used; the lowest inhibition zone was found at 20% concentration with a measurement of 10 mm, while the biggest inhibition zone was measured at 18 mm and increased to 23 mm at 80% and 100% concentrations, respectively. DMSO was used as a control which give a negative effect against Pseudomonas aeruginosa as shown in figure 5.

Figure 4. The anti-microbial activity of Eucalyptus oil on P. aeruginosa

Figure 5. The negative effect of DMSO as a Control against P. aeruginosa
DISCUSSION
Some references showed that Azithromycin was not approved for the treatment of infection caused by P. aeruginosa, and there are no published breakpoints for this species. Still, other references showed that the direct effect of Azithromycin was on the outer membrane of P. aeruginosa, which might contribute to its bactericidal activity against this organism. P. aeruginosa is not only naturally resistant to a wide variety of anti-microbials but also possesses an extraordinary capacity for developing resistance to commonly used anti-microbials through the selection of mutations in chromosomal genes or by horizontally acquiring resistant determinants. P. aeruginosa is resistant to anti-microbials not only because it is naturally resistant to them, but also because it has an extraordinary capacity to develop resistance to commonly used anti-microbial Amikacin and colistin were shown to be the most effective antibiotics against blood. Respiratory P. aeruginosa isolates, according to certain investigations 6 ,19 , Our findings were in contrast to those of earlier research. Given that two of our country’s isolates showed resistance to Amikacin, we suspect this disparity may be attributable to inappropriate antibiotic prescribing practices. By attaching to the 30S subunit of the ribosome, the aminoglycoside antibiotics gentamicin and Amikacin can disrupt the process of protein synthesis. The low permeability of P. aeruginosa’s cell wall, particularly its outer membrane, is generally thought to be the cause of the bacteria’s intrinsic resistance to all antibiotics. Our results of sesame oil antibacterial activity have differed from another researcher’s since 20 showed that sesame oil exhibited strong anti-microbial effects against the tested bacteria with inhibition zone ranging from 15 to 25 mm. This could be because the organism used for testing was pseudomonas aeruginosa, which is highly resistant to many antibiotics and anti-microbial agents. Additionally, the sesame oil that we used came from the market, and it’s possible that it lost some of its activity because of improper storage because it was imported from Iran. Methanol extracts of different parts of Sesame Indicum l. (root, seed, and leaves) had different levels of anti-oxidant and anti-microbial activity. This was assayed by agar disc diffusion and agar well diffusion method against five bacterial species. Also, these results disagreed with our results, which may be due to the use of imported sesame oil from other countries rather than sesame oil derived from local products 21. A previous study performed by Yepola and Adeniyi 22 showed that phytochemical screening of leaf extracts of Eucalyptus tannins, saponins, and cardiac glycosides was discovered in camaldulensis after testing the plant. Also, this demonstrated that the methanol extract, dichloromethane fraction, and methanol residue represent a broad spectrum of activity; the methanol extracts demonstrated greater activity against Salmonella typhi, Staphylococcus aureus, and Bacillus subtilis (15-16 mm) than they did against Klebsiella ssp., Yersinia enterocolitica, and Pseudomonas aeruginosa (14 mm). The findings of our research were consistent with those of a previous study. The essential oils extracted from Eucalyptus globulus leaves have a density lower than that of water, a hue similar to a pale yellow, and an aromatic scent that is spicy. The antibacterial activity of essential oil displayed considerable against different strains of Staphylococcus aureus that were tested. However, the antibacterial activity of essential oil remained lower than that of aqueous extract, which showed the highest antibacterial activity. Bowras and his coworker showed this. Our findings regarding the antibacterial activity of eucalyptus oil against ten clinical isolates of Pseudomonas aeruginosa coincide with the literature in that a higher oil concentration had a more potent effect on Pseudomonas aeruginosa than a lower concentration of the oil did1. On the other hand, Damjanovic – Vrantica et al. reported that the anti-microbial activity test demonstrated that the essential oil of E. globulus possesses a rather potent anti-microbial activity, particularly against Streptococcus pyogenes, E. coli, Candida albicans, Staphylococcus aureus, Acinetobacter baumanni, and Klebsiella pneumoniae, with the exception of Pseudomonas aeruginosa &Salmonella infantis 23. Pseudomonas spp. are recognized to have the ability to metabolize a wide range of organic chemicals, and because of this property, it is utilized extensively in bioremediation. This may be the reason for their high-level resistance. Chao’s team 24 demonstrated this. This bacterium may be simplifying and metabolizing the chemicals in the oils that are inhibitory to many of the other bacteria. This would be beneficial to the overall bacterial population. The primary bioactive compounds in Eucalyptus were -pinene, p-cymene, limonene, and –terpinene 25. According to the researchers’ findings, the essential oils of rosemary and Eucalyptus have the highest levels of 1,8-cineole of any other essential oils. Another study investigated the efficacy of various essential oils against multidrug-resistant strains of P. aeruginosa. The essential oils investigated included Cinnamomum zylanicum (Dalchini oil), Eucalyptus globulus (Nilgri oil), Eugenia caryophyllata (Clove oil), Ocimum sanctum (Tulsi oil), and Allium sativum (Garlic oil). Compared to other oils, the inhibitory action of Cinnamomum zylanicum oil against multidrug-resistant bacteria was shown to be the most potent, followed by the inhibitory action of Eucalyptus globulus oil (Nilgri oil).
CONCLUSIONS
Pseudomonas aeruginosa is an opportunistic pathogen. Infections by P. aeruginosa are challenging to treat since these bacteria can acquire resistance to different antibiotics. Our findings conclude that eucalyptus oil may be used in the treatment as an external ointment for wound infection by Pseudomonas aeruginosa as an alternative or along with antibiotics
Acknowledgment: Thanks going to all who support us.
The conflict between authors: No conflict
Funds: self by authors
REFERENCE
1. Bouras M, Abbaci NB, Bennadja S. Antibacterial Activity of Essential Oil and Aqueous Extract of Eucalyptus globulus Against Methicillin Resistance Staphylococcus aureus and Methicillin Sensitive Staphylococcus aureus. International Journal of Pharmacognosy and Phytochemical Research 2016; 8(10); 1717-1721.
2. Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Current opinion in microbiology. 2009 Feb 1;12(1):61-6.
3. Breidenstein EB, de la Fuente-Núñez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends in microbiology. 2011;19(8):419-26.
4. Takahashi T, Kokubo R, Sakaino M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Letters in applied microbiology. 2004;39(1):60-4.
5. Hussein NN, Muslim AH. Detection of the antibacterial activity of AgNPs biosynthesized by Pseudomonas aeruginosa. The Iraqi Journal of Agricultural Science. 2019;50(2):617-25.
6. Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodríguez C, Moya B, Zamorano L, Suárez C, Peña C, Martínez-Martínez L, Oliver A. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Anti-microbial agents and chemotherapy. 2011;55(5):1906-11.
7. Pitout JD, Revathi G, Chow BL, Kabera B, Kariuki S, Nordmann P, Poirel L. Metallo-β-lactamase-producing Pseudomonas aeruginosa isolated from a large tertiary centre in Kenya. Clinical Microbiology and Infection. 2008;14(8):755-9.
8. Manika N, Chanotiya CS, Negi MP, Bagchi GD. Copious shoots as a potential source for the production of essential oil in Eucalyptus globulus. Industrial Crops and Products. 2013;46:80-4.
9. Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Anti-microbial agents and chemotherapy. 2004;48(8):3086-92.
10. Mustafa MA. Effect of Eucalyptus leaves and its supplementation with diet on broiler performance, microbial and physiological statues to alleviate cold stress. Iraqi Journal of Agricultural Sciences. 2019;50(1).
11. Musyimi DM, Ogur JA. Comparative assessment of antifungal activity of extracts from Eucalyptus globulus and Eucalyptus citriodora. Research Journal of Phytochemistry. 2008;2(1):35-43.
12. Rios JL, Recio MC. Medicinal plants and anti-microbial activity. Journal of ethnopharmacology. 2005;100(1-2):80-4.
13. Laj S, Bankole MA, Ahmed T, Bankole MN, Shittu RK, Saalu CL, Ashiru OA. Antibacterial and antifungal activities of essential oils of crude extracts of Sesame radiatum against some common pathogenic microorganisms. Iranian Journal of Pharmacology & Therapeutics. 2007;6:165-170.
14. HASAN AF, BEGUM S, Furumoto T, FUKUI H. A new chlorinated red naphthoquinone from roots of Sesamum indicum. Bioscience, biotechnology, and biochemistry. 2000 Jan 1;64(4):873-4.
15. Cooney RV, Custer LJ, Okinaka L, Franke AA. Effects of dietary sesame seeds on plasma tocopherol levels. Nutrition and cancer. 2001 Jan 1;39(1):66-71.
16. Jaradat NA, Zaid AN, Abuzant A, Shawahna R. Investigation the efficiency of various methods of volatile oil extraction from Trichodesma africanum and their impact on the anti-oxidant and anti-microbial activities. Journal of Intercultural Ethnopharmacology. 2016 Jun;5(3):250-256.
17. Head SW, Swetman AA, Hammonds TW, Gordon A, Southwell KH, Harris RV. Small scale vegetable oil extraction. Small scale vegetable oil extraction.. 1995. Natural Resources Institute, Chatham, UK. ISBN 0859543870; http://gala.gre.ac.uk/id/eprint/12074
18. OO A, Adeniyi BA. The antibacterial activity of leaf extracts of Eucalyptus camaldulensis (Myrtaceae). Journal of Applied Sciences Research. 2008;4(11):1410-3.
19. Sader HS, Farrell DJ, Flamm RK, Jones RN. Anti-microbial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagnostic microbiology and infectious disease. 2014;78(4):443-8.
20. Saleem TM. Anti-microbial activity of sesame oil. Int J Phytochem Pharmacol. 2011;1(1):21-3.
21. Sandhya S, Priti G, Ashwani K, Jaswant R, Bipin KA, Pankaj G, Anubhuti S. In vitro evaluation of roots, seeds and leaves of Sesamum indicum L. for their potential antibacterial and anti-oxidant properties. African Journal of Biotechnology. 2014;13(36):3692-701.
22. Tawfiq SM. Bacteriologigal and genetic study of Pseudomonas aeruginosa isolates. Iraqi Journal of Agricultural Sciences. 2018;49(1). https://doi.org/10.36103/ijas.v49i1.201
23. Damjanović-Vratnica B, Đakov T, Suković D, Damjanović J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech Journal of Food Sciences. 2011;29(3):277-84.
24. Chao SC, Young DG, Oberg CJ. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. Journal of essential oil research. 2000;12(5):639-49.
25. Statistics IS. IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. Armonk, NY: IBM Corp.
Received: April 22, 2022 / Accepted: June 18, 2022 / Published:15 November 2022
Citation: Khalaf R J, Hussein A R. Antibiogram of Eucalyptus and Sesame seed oil against clinical isolates of Pseudomonas aeruginosa.Revis Bionatura 2022;7(4) 61. http://dx.doi.org/10.21931/RB/2022.07.04.61




















