Vol 7 No 4 2022- 30

Effects of Leptin antagonist treatments on testosterone and testis histological characteristics of immature male mice
Mohammed A. Kh. Al-Aqbi1
1College of Agriculture, Wasit University, Wasit, Iraq
Corresponding author e-mail moabdalah@uowasit.edu.iq
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.30
ABSTRACT
The present study aimed to ascertain how leptin antagonist injection affected testis weights, testis morphology and testosterone levels in immature male Swiss mice. Animals were administered with anti-leptin antibody subcutaneously, with or without equine chorionic gonadotropin (eCG). Control animals were treated with non-immune serum. Blood and testis were collected. The Androgen profile was analyzed in serum and tissue homogenates, and testes were histologically examined. Compared to controls, mice treated with an anti-leptin antibody with or without gonadotropins had a significant (p<0.05) increase in testis weight. Testosterone concentrations in the testis were significantly (p<0.05) higher in mice administered with anti-leptin antibody compared to control, but testosterone concentrations in blood were not affected. The diameter of seminiferous tubules, the diameter of the lumen and the width of spermatogenic cells were significantly (p<0.05) higher in mice in treatment groups compared to controls. We conclude that anti-leptin antibody administration in immature male mice increased testosterone concentrations in the testis and improved testis histological characteristics.
Keywords: leptin; mouse; histology; testis; testosterone; immature male
INTRODUCTION
The testis is an essential sexual organ in male animals. Testicular growth directly influences semen quality in humans and male mammals, including rats and boars, which is necessary for reproductive functions 1, 2. Testicular development occurs primarily in the seminiferous tubules after birth, including the Sertoli cell, germ cell, and Leydig cell proliferation. Most human testes development occurs during adolescence between the ages 2 to 14 years 3. Testis volume, testis weight, Sertoli cells, and testosterone levels in the testes gradually rise from 2 to 8 years of age, and the number of germ cells grows by 3 to 6 times 4. As a result, the spermatogonial cells proliferate rapidly.
In late 1994, a significant publication by Zhang et al. on the structure of the mouse obese (ob) gene and its human equivalent using positional cloning was described 5. The ob gene results in the protein known as leptin. The ob/ob mouse’s obesity is caused by a gene mutation that prevents leptin from being secreted by adipocytes, which leads to obesity. Leptin reduces food intake and increases energy expenditure, which leads to a loss in body weight in mice 6, 7. Additionally, ob gene expression is elevated in many animal models of obesity 8 and human obesity 9. Leptin may be crucial for regulating body weight, according to accumulating research. The ob/ob mouse has atrophic reproductive organs and is infertile 10. Much like in prepubertal animals, gonadotropin secretion is impaired and extremely sensitive to the negative feedback of gonadal steroids in the ob/ob mouse 11. It has been demonstrated that long-term administration of leptin can restore fertility and the growth and function of the reproductive system in ob/ob mice 12 by boosting the release of gonadotropins 13. According to the crucial weight hypothesis, puberty starts when body weight reaches a certain threshold 14. Since underfed rats postpone puberty, the original premise is untrue. However, when given access to food, rapid weight gain causes rats to reach puberty at weights far lower than the weight required for normal nutritional conditions 15. As a result, when body fat comes to a specific level, puberty is likely to occur 14, 15.
By altering kisspeptin production in the arcuate nucleus, leptin can indirectly control gonadotropin secretion from the hypothalamus 16. In addition to its stimulatory actions on the hypothalamus, leptin directly affects the anterior pituitary 17. Gonadotropin-releasing hormone (GnRH), which is produced by the hypothalamic-pituitary-gonadal axis and regulates the activity of the testicles during reproduction, stimulates the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH) 18. Luteinizing hormone and FSH control steroidogenesis and spermatogenesis in the testis 19. Leptin is present in men’s spermatocytes, and high levels of leptin in the testicles have been associated with defective spermatogenesis 20. Male fertility may be hampered by obesity. In humans and rodents, obesity can impair spermatic function and reduce sperm motility, viability, and concentration 21, 22. Obese people have a higher BMI and higher leptin levels, contributing to poor sperm quality 23, lower sperm counts 24, and a higher DNA fragmentation index 25. Leptin and leptin receptor levels are higher in infertile males, implying that leptin has local effects on spermatogenesis and testis function 26. This study aimed to see how antagonist leptin affects testis histology and testosterone levels in immature male mice.
MATERIALS AND METHODS
Animal Maintenance
Twenty immature male Swiss random-bred male mice (3 weeks old) with an average weight of 15–20 g were used in this experiment. Mice were housed in an air-conditioned room under a 12-h light-dark cycle (lights on 0700–1900 hours). Water and food in the form of standard pellets were given ad libitum to the mice. Wood shavings were used as bedding, covering cages’ bottoms to absorb urine. The bedding would be changed on average every three days to maintain a clean environment for the mice and reduce unnecessary infection.
Experimental Design
Four groups of mice (n = 5/group) were given subcutaneous (sc) injections (100 µl) every 48 h of the following treatments for three times: (i) non-immune Ig (50 µg) as the control group; (ii) anti-leptin antibody (JMCK#16, 50 µg); (iii) ECG (40 IU) with non-immune Ig (50 µg) and (iv) anti-leptin antibody (50 µg) with ECG (40 IU). On the morning of the day, 6 of treatment (0900 h), animals were euthanized by CO2 asphyxiation and testes, dissected and weighed, and blood was collected. Testes were stored at –20oC for homogenization or fixed in Bouin’s solution.
Tissue and Sample Preparation
Testis tissues were homogenized on iv in a handhold homogenizer (IKA® T 10 Basic) for 30 seconds in homogenizing buffer containing (EDTA 5mMol/L, EGTA 5mMol/L, and 0.02% sodium azide). Homogenates were stored at -20°C for required to assay hormones. Mice were euthanized by CO2 asphyxiation, and blood was collected immediately and allowed to clot for 30 minutes before being centrifuged and the serum removed and frozen at -20C. The testes were weighed and placed in 5ml polypropylene vials (Tube 5ml*16pp+Cap NAT). In each animal, one testis was put on ice and then stored at -20C until processed further, and the other testis was fixed in Bouin’s solution for 48 hours at room temperature. All the animal experimentation was approved by the University of New England Animal Ethics Committee and is under the NH&MRC Code of Practice for the Care and Use of Animals for Experimental Purposes.
Steroid Assays
Testosterone concentration in serum or tissue homogenate was determined in duplicate by LC/MS/MS using the method of 27 with minor modifications. A Shimadzu UPLC and 8050 triple quadrupole mass spectrometer equipped with a heated electrospray ionization source (HESI) operating in positive ion mode were used. The samples were separated using a Kintetex 2.6u Evo C18 column (2.1 x 50mm 2u particle size) (Phenomenex). Samples were eluted with a gradient from 10% to 100% methanol with 0.2mM ammonium fluoride over 6 minutes. Testosterone was eluted with a retention time of 2.1 and 2.5 minutes, respectively and quantitative analysis was performed in multiple reaction monitoring modes (MRM) of the two most abundant product ions (m/z 361 > 163.1 & 121.1 and 363.2 > 121.2 & 309.3). The inter and intra-assay coefficients of variation were determined to be 4.9% and 2.7%, respectively. The sample results were calculated in serum as pg/ml and pg/mg in tissue homogenates.
Testis Histology
The abstracted testis was fixed in Bouin’s solution for 48 hours at room temperature. Fixed testes were trimmed transversely into three parts. The middle part was then immersed in 70% alcohol, followed by immersions in a series of alcohol solutions with ascending concentrations. After dehydration, the tissue samples were processed further before being sectioned using a rotary microtome. A small drop of Mayer’s Albumin was placed at the center of the glass slide and spread evenly using a cleaned finger. A drop of distilled water was then placed on the same glass slide, and tissue sections were transferred onto the slide. The glass slides were dried and kept in a slide box. The hematoxylin and eosin (H&E) staining technique would stain the nucleus purple and the cytoplasm pink. The steps of the H&E staining technique included deparaffinization, hydration, hematoxylin and eosin staining, dehydration, and clearing. The features evaluated were the diameter of seminiferous tubules, the diameter of the lumen, and the width of the spermatogonia layer, spermatocytes layer, and spermatid-sperm layer.
Statistical analysis
Two-way analysis of variance followed by the Student-Newmen-Keules multiple range tests was performed using the SAS computer software package (SAS Institute Inx., Cary, NC USA). Data are presented and expressed as means ± standard error of the mean (SEM). Unless otherwise stated, p < 0.05 was considered significant.
RESULTS
Testis weights
The relative testicular weights (mg) are shown in figure1. The testicular weights of mice treated with anti-leptin, ECG and anti-leptin with ECG (96.29± 7.32 mg, 99± 11.66 mg and 132.63± 12.92 mg, respectively) were significantly (p< 0.05) heavier than the control group (74.87± 5.04 mg). The anti-leptin with ECG group was considerably more severe than the anti-leptin and ECG groups.
Testis Histological characteristics
Relative testis histological characteristics of the mature male mice treated with anti-leptin, ECG, anti-leptin with ECG and the control group are shown in figure 2 and table 1. The seminiferous tubules lumen diameter was significantly (p<0.05) more significant in anti-leptin(199.4±11.29 𝜇m), ECG(207.2±13.04 𝜇m), and anti-leptin with ECG ( 219.4±16.45 𝜇m ) than the control group (187.7±12.14 𝜇m).
The diameter of seminiferous tubules lumen of mice treated with anti-leptin and anti-leptin with ECG groups (51.6±5.18 𝜇m and 53.9±9.88𝜇m respectively) was significantly (p<0.05) more significant than the control group (49.3±5.85 𝜇m).
The width of the spermatogonia layer of mice treated with anti-leptin, ECG and anti-leptin with ECG (19.2±5.33 𝜇m, 20.1±4.74 𝜇m and 22.8±6.38 𝜇m respectively) was significantly (p<0.05) more significant than the control group (17.1±4.24 𝜇m), while were no significant (p<0.05) differences between anti-leptin group and eCG group.
The width of the spermatocytes layer of mice had no significant (p<0.05) differences between the anti-leptin group (29.4±4.87 𝜇m) and control group (30.1±4.37 𝜇m). However, the width of the spermatocytes layer of mice treated with eCG and anti-leptin with eCG (33.2±6.24 𝜇m and 33.5±9.55 𝜇m respectively) was significantly more significant than the control group.
The width of the spermatid-sperm layer of mice treated with anti-leptin, eCG and anti-leptin with eCG (24.9±3.58 𝜇m, 25.3±5.30 𝜇m and 27.8±7.94 𝜇m respectively) was significantly (p<0.05) more significant than the control group (23.2±2.26 𝜇m).
Testosterone levels in serum and testis
Testosterone concentrations in the serum of immature male mice treated with anti-leptin, eCG, anti-leptin with eCG and the control group are shown in Figure 3 (a). Testosterone concentrations in the serum of mice treated with anti-leptin and control group were significantly (p<0.05) lower than eCG and eCG supplemented with anti-leptin groups. Testosterone concentrations in the serum of mice treated with eCG (4.47± 1.46 ng/ml) were the highest and more significant than the mice that were treated with eCG supplemented with anti-leptin (3.92± 1.32 ng/ml), control group (0.77± 0.14 ng/ml) and anti-leptin (1.02± 0.22 ng/ml).
Testosterone concentrations in the testis of treatment groups were significantly (p<0.05) higher than the control group (Fig.3b). Testosterone concentrations in the testis of mice treated with eCG supplemented with anti-leptin (527.69±0.88 ng/testis) were significantly higher than control group (63.28±0.35 ng/testis), anti-leptin group (237.77±0.18 ng/testis) and eCG group (242.87±0.11 ng/testis).
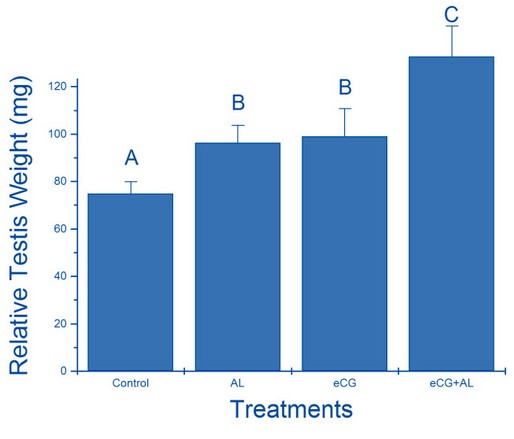
Figure 1. The relative effect of treatment with anti-leptin (AL), eCG, and a combination of AL and eCG on testis weight.

Table 1. Testis histological characteristics (mean ± SE).

Figure 2. Seminiferous tubules (200x magnification) for (a) C group, (b) anti-leptin (AL) group, (c) eCG group, and (d) eCG+AL group. Note L = lumen of the seminiferous tubule, and SC = spermatogenic cells.

Figure 3. (a) The relative effect of treatment with anti-leptin (AL), eCG, and a combination of AL and eCG on testosterone concentrations in serum; (b) The relative effect of treatment with anti-leptin (AL), eCG, and a combination of AL and eCG on testosterone concentrations in testis.
DISCUSSION
Leptin, an ob gene hormone released by adipocytes, is a key player in weight control and serves as a neuroendocrine mediator for reproductive function 17, 28. Leptin acts on the central nervous system (CNS) as a neuroendocrine hormone to modulate the HPG axis’s production as a nutritional signal 29. Leptin seems to play a role in both males’ and females’ onset of puberty and the maturation of their reproductive systems 30, 31. Compared to controls, leptin administration to ob/ob male mice boosted serum levels of FSH and increased testicular weights. In contrast, leptin administration had an inhibitory effect on reproduction in normal males 13. Exogenous leptin treatment is detrimental to testis morphology and sperm count in normal rats 32, 33, and in normal mice 34. The contribution of leptin to the function of the male reproductive system has been less clear. Therefore, in the present work, we investigated for the first time the effects of in vivo anti-leptin treatment on testis morphology and testosterone concentrations in normal immature mice. The current results show testis weights in mice-treated groups were significantly heavier than in the control group.
Moreover, our study showed that the testicular testosterone concentrations were significantly higher in anti-leptin-treated mice than in the control group. These results suggest that anti-leptin treatment positively affects testis weights and testosterone concentrations in the testis. These results correspond with prior studies, which showed that leptin inhibits the reproductive axis 33, 35, claiming that reducing leptin could stimulate reproductive function. Moreover, testosterone concentrations in the testis of eCG with anti-leptin treated mice were significantly higher compared to anti-leptin, eCG and control groups. Anti-leptin and eCG treatment have a synergetic effect on the local concentrations of testosterone in the testis of immature male mice, which increase testosterone concentrations, suggesting that anti-leptin treatment increase the consumption of testosterone that is used for reproductive activities. This hypothesis could explain the non-significant differences in circulating testosterone concentrations between anti-leptin-treated mice and control. Leptin appears to negatively impact male fertility when serum leptin levels are higher than usual in lean mice treated with exogenous leptin. According to our research, anti-leptin treatment induces and increases testis weight and gives better testis histological characteristics in immature mice.
The exogenous treatments of anti-leptin to normal immature male mice increased the diameter of seminiferous tubules and the diameter of the lumen compared to the control. In addition, rustles also showed improvement in the width of spermatogenic cells, which includes the spermatogonia layer, spermatocytes layer and spermatid-sperm layer in the seminiferous tubules. In all of the previous layers, increasing their thickness has been shown in our results. To maintain ongoing male fertility, the testes continuously create millions of spermatozoa each day during steady-state spermatogenesis 36. The self-renewal and differentiation of spermatogonial stem cells, a tissue-specific stem cell population recognized in mammals, are necessary for this process 37, 38. Testis weights and seminiferous tubule diameter were increased by the reduction of leptin levels in normal immature male mice. The reproductive pathways of the male mouse are significantly influenced by leptin 39. The results of testis histological characteristics research revealed the process of spermatogenesis. Spermatogenesis and male fertility are maintained in part by testosterone signaling 40. In hypothesis, the reduction of leptin in males induces Leydig cells to produce more testosterone in the testis. Leydig cells located in the interstitial compartment are the source of testosterone in the testes. Therefore, it is not surprising that testicular testosterone levels are much higher than blood serum levels 41, suggesting a boost of sperm counts and testosterone concentrations in the testis and improving fertility in immature mice. Moreover, results also showed a synergistic effect of anti-leptin and eCG on the diameter of seminiferous tubules, the diameter of the lumen, and the width of spermatogenic cells. All these parameters were significantly higher in treatment groups compared to controls. eCG is known to have LH-like action 42, leading to increased testosterone. Baker et al. demonstrated that dihydrotestosterone (DHT) is the primary active androgen in immature testis, whereas, in mature testis, the dominant androgen is testosterone 43. Dihydrotestosterone is converted to testosterone by testicular 5α-reductase activity 44. The increased weight of testis in mice treated with anti-leptin plus eCG may be attributed to the action of DHT and mesenchymal/ epithelial interactions.
CONCLUSIONS
Our study implicates the effect of leptin antagonist treatments on testosterone and testis histological features of immature male mice. The treatment of anti-leptin with or without eCG in juvenile male mice improves testis histological features and increases testis weights and testicular testosterone concentrations.
Funding: This research received no external funding.
Institutional Review Board Statement: All animal experimentation was approved by the University of New England Animal Ethics Committee and followed the NH&MRC Code of Practice for the Care and Use of Animals for Experimental Purposes.
Data Availability Statement: This study’s study data and materials are available upon request.
Acknowledgments: The author acknowledges the assistance of the school of Science and Technology at the University of New England, Armidale, NSW, Australia. Thanks to Professor James R McFarlane for his collaboration in the research process.
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
REFERENCES
1. Shomali T, Taherianfard M, Dalvand M, Namazi F. Effect of pharmacological doses of niacin on testicular structure and function in normal and diabetic rats. Andrologia. 2018;50(10):e13142.
2. Flowers W. Factors affecting the production of quality ejaculates from boars. Animal Reproduction Science. 2021:106840.
3. Chamindrani Mendis-Handagama S, Siril Ariyaratne H. Differentiation of the adult Leydig cell population in the postnatal testis. Biology of reproduction. 2001;65(3):660-671.
4. Chen H, Ge R-S, Zirkin BR. Leydig cells: from stem cells to aging. Molecular and cellular endocrinology. 2009;306(1-2):9-16.
5. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. nature. 1994;372(6505):425-432.
6. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540-543.
7. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543-546.
8. Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochemical and biophysical research communications. 1997;231(1):26-29.
9. Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Paper presented at: Seminars in vascular medicine, 2005.
10. Swerdloff R, Batt R, Bray G. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359-1364.
11. SWERDLOFF RS, PETERSON M, VERA A, Batt R, HEBER D, BRAY GA. The hypothalamic-pituitary axis in genetically obese (ob/ob) mice: response to luteinizing hormone-releasing hormone. Endocrinology. 1978;103(2):542-547.
12. Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Molecular human reproduction. 1997;3(6):467-472.
13. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144-3147.
14. Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185(4155):949-951.
15. Ronnekleiv OK, Ojeda SR, McCann SM. Undernutrition, puberty and the development of estrogen positive feedback in the female rat. Biology of Reproduction. 1978;19(2):414-424.
16. Gottsch M, Cunningham M, Smith J, Popa S, Acohido B, Crowley W, Seminara S, Clifton D, Steiner R. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073-4077.
17. Yu W, Kimura M, Walczewska A, Karanth S, McCann S. Role of leptin in hypothalamic–pituitary function. Proceedings of the National Academy of Sciences. 1997;94(3):1023-1028.
18. Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J-M. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398.
19. Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reproductive biology. 2013;13(1):1-14.
20. Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia. 2007;39(1):22-27.
21. Fernandez C, Bellentani FF, Fernandes G, Perobelli JE, Favareto A, Nascimento AF, Cicogna AC, Kempinas W. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32.
22. Vigueras-Villaseñor RM, Rojas-Castañeda JC, Chavez-Saldana M, Gutiérrez-Pérez O, García-Cruz ME, Cuevas-Alpuche O, Reyes-Romero MM, Zambrano E. Alterations in the spermatic function generated by obesity in rats. Acta histochemica. 2011;113(2):214-220.
23. Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Human reproduction. 2005;20(1):208-215.
24. Jensen TK, Andersson A-M, Jørgensen N, Andersen A-G, Carlsen E, Skakkebæk NE. Body mass index in relation to semen quality and reproductive hormonesamong 1,558 Danish men. Fertility and sterility. 2004;82(4):863-870.
25. Kort HI, Massey JB, Elsner CW, Mitchell‐Leef D, Shapiro DB, Witt MA, Roudebush WE. Impact of body mass index values on sperm quantity and quality. Journal of andrology. 2006;27(3):450-452.
26. Chen B, Guo JH, Lu YN, Ying XL, Hu K, Xiang ZQ, Wang YX, Chen P, Huang YR. Leptin and varicocele‐related spermatogenesis dysfunction: animal experiment and clinical study. International journal of andrology. 2009;32(5):532-541.
27. Ionita IA, Fast DM, Akhlaghi F. Development of a sensitive and selective method for the quantitative analysis of cortisol, cortisone, prednisolone and prednisone in human plasma. Journal of Chromatography B. 2009;877(8-9):765-772.
28. Chehab FF. The reproductive side of leptin. Nature medicine. 1997;3(9):952-953.
29. Zak L, Cosgrove J, Aherne F, Foxcroft G. Pattern of feed intake and associated metabolic and endocrine changes differentially affect postweaning fertility in primiparous lactating sows. Journal of Animal Science. 1997;75(1):208-216.
30. Huang L, Cai L. Leptin: a multifunctional hormone. Cell research. 2000;10(2):81.
31. Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertility and sterility. 2002;77(3):433-444.
32. Almabhouh F, Osman K, Siti Fatimah I, Sergey G, Gnanou J, Singh H. Effects of leptin on sperm count and morphology in Sprague‐Dawley rats and their reversibility following a 6‐week recovery period. Andrologia. 2015;47(7):751-758.
33. Haron MN, D’Souza UJ, Jaafar H, Zakaria R, Singh HJ. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertility and sterility. 2010;93(1):322-324.
34. Wang X, Zhang X, Hu L, Li H. Exogenous leptin affects sperm parameters and impairs blood testis barrier integrity in adult male mice. Reproductive Biology and Endocrinology. 2018;16(1):55.
35. Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. European journal of endocrinology. 2000;143(3):293-311.
36. Sharpe R. Regulation of spermatogenesis. The physiology of reproduction. 1994;1:1363-1434.
37. DE Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. Journal of andrology. 2000;21(6):776-798.
38. Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annual review of cell and developmental biology. 2008;24:263.
39. Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190-1193.
40. Walker WH. Non-classical actions of testosterone and spermatogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1546):1557-1569.
41. Turner T, Jones C, Howards S, Ewing L, Zegeye B, Gunsalus G. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115(5):1925-1932.
42. Min K-S, HIYAMA T, SEONG H-H, HATTORI N, TANAKA S, SHIOTA K. Biological activities of tethered equine chorionic gonadotropin (eCG) and its deglycosylated mutants. Journal of Reproduction and Development. 2004;50(3):297-304.
43. Baker H, Bailey D, Feil P, Jefferson L, Santen R, Bardin C. Nuclear accumulation of androgens in perfused rat accessory sex organs and testes. Endocrinology. 1977;100(3):709-721.
44. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011;32(1):81-151.
Received: August 25, 2022 / Accepted: October 12, 2022 / Published:15 November 2022
Citation: Al-Aqbi, Mohammed. Effects of Leptin antagonist treatments on testosterone and testis histological characteristics of immature male mice. Revis Bionatura 2022;7(4) 30. http://dx.doi.org/10.21931/RB/2022.07.04.30



















