Vol 9 No 1 2024-51
2024..09.01.51
Identification of Diagnosis Fungi that Cause Potato Root Rot
Saja W. A´shour1 and Theyab A. Farhan2
1Department of Plant Protection, College of Agriculture, University of Anbar, Iraq. saj20g7013@uoanbar.edu.iq.
2Department of Plant Protection, College of Agriculture, University of Anbar, Iraq. deab.frahen@uoanbar.edu.iq
* Correspondence: saj20g7013@uoanbar.edu.iq.
Available from. http://dx.doi.org/10.21931/RB/2024.09.01.51
ABSTRACT
Results of collecting samples from different regions of Anbar Governorate (Al-Amiriyah, Al-Khalidya, Fallujah, Heet and Ramadi) showed that potato root rot disease is widespread in all collected areas. The isolation and phenotypic and molecular diagnosis results using the polymerase chain reaction (PCR) technique indicated the presence of fungus Rhizoctonia spp. and Fungus Fusarium spp. Accompanying potato root rot disease and the pathogenicity test using radish seeds on water Agar (W.R.) culture media, all tested isolates achieved a significant reduction in radish seed plants compared with control treatment uncontaminated by any of the isolates of fungi, which recorded infection rate 0%.
Keywords: Potato Root Rot, Diagnosis, Fungi, Rhizoctonia solani , Fusarium solani.
INTRODUCTION
Potato, Solanum tuberosum L., is considered a strategic vegetable crop and comes in fourth place after wheat, corn and rice. It is from the family Solanaceae, of strategic importance in most countries of the world in general and Iraq in particular, as it is considered one of the easiest crops to manage when compared to other vegetable crops as well as Most of its varieties, especially the early ones, complete their life cycle less than 100 days 1. Due to the ease of management of this crop and its high productivity and short life cycle, its cultivation has expanded significantly 2. As a result of the expansion in the cultivation of this crop, many problems appeared, foremost of which are fungal causes, especially root rot pathogens, leg ulcers and black crust, which cause economic losses. It increases farmers’ production 3, 4 . Root rot disease is one of the most essential plant diseases worldwide that affects many crops 5. Root rot disease of potato plants caused by the fungi Rhizoctonia solani and Fusarium solani is among the most common diseases due to its highly destructive effects that may reach chronic effects in every potato growing area. Each of them can have an economic impact on the season and does not cause Economic losses are only on an annual basis and therefore considered a problem for producers who often suffer economic losses from this disease. Mold was first described as a potato tuber disease in Ireland in 1913, has been known to occur in North America since then, and has become a significant soil-borne disease. Statistics show that this species is becoming increasingly common, which poses a considerable threat to potato production, especially in warm regions 6. Root rot symptoms represent a significant threat because the damage begins underground, where the first symptoms cannot be distinguished—the appearance of pathological symptoms on infected plants 7, 8. Because of the importance of the potato crop and the danger of root rot disease, the study aimed to isolate and characterize the phenotypic and molecular diagnosis of the fungi accompanying the samples infected with root rot disease.
MATERIALS AND METHODS
Sample collection
Samples were collected from potato plants that showed symptoms of disease (yellow, plant wilt, plant death, burn edges of leaves) 9 from some fields of Anbar Governorate (Al-Khalidiya, Al-Amiriya, Sadat Al-Fallujah, Nuaimiya, Heet and Saqlawiyah) for the agricultural season Two thousand twenty-one for the spring loop. The affected plant parts were placed in sterile polyethylene bags, with the place and date of sample collection recorded. The samples were kept in the refrigerator at a temperature of 4 C° until the isolation.
Isolation of fungi associated with potato root rot
Parts of roots that showed symptoms of rot were taken and washed with water to get rid of dust and cut into small pieces with a length of 0.5-1 cm. and superficially sterilized with sodium hypochlorite solution Nacl (chlorine 6% active substance) for 3 minutes after which the plant pieces were washed with distilled water Sterilized 3 times to get rid of the effects of the sterile material, dried with sterile filter paper and transferred by sterile forceps, and 4 plant pieces were planted in each Petri dish with a diameter of 9 cm containing the sterilized culture medium (PDA) Potato Dextrose Agar with an Autoclaved at 121 °C and a pressure of 5.1 bar/cm3 for 20 minutes and the antibiotic (Amoxicillin) was added to it at a concentration of 200 mg. L-1, after sterilizing medium. The dishes were incubated at 25 ± 2 °C for 3 days, after which a microscopic examination was conducted to investigate the presence of pathogenic fungi.
Phenotypic diagnosis
Fungi isolates were diagnosed after the growth of fungal colonies on the PDA medium was prepared, as microscopic slides were examined using a light microscope, and depending on the cultural characteristics and using the taxonomic key specific to the species, the fungi were morphologically diagnosed 10.
Molecular diagnosis
DNA was isolated and extracted in the Scientific Progress Laboratory / Baghdad – Al-Harithiya according to the ABIOpure protocol. Prepared by the Korean company Macrogen ITS13- 5′- TCCGTAGGTGAACCTGCGG, ITS43 – 5′–TCCTCCGCTTATTGATATGC. The Korean company Macrogen prepared the primers in lyophilized form in nuclease-free water to give a final concentration of 100 μl.μl -1. A working solution of these primers was prepared by adding 10 μl of the starting primer (stored at -20 °C) to 90 μl of distilled water to obtain an effective starting solution of 10 μl. mol-1 After PCR, gel electrophoresis to confirm the presence of amplification using polymerase chain reaction (PCR) was fully approved on the parameters of the extracted DNA. After completing the DNA amplification and migration steps on Agarose Gel, the samples were sent to the Korean Macrogen Company to obtain the sequence of nitrogenous bases (Sequencing). Several studies have relied on this technique in the diagnosis of fungi, as 11 showed that the PCR technique is used to diagnose R. solani fungi and relied in its diagnosis on the transcribed spacer internal region between (ITS4-ITS1) to distinguish between types of fungi specialized. 12 Also used PCR techniques to diagnose species of the genus Rhizoctonia, such as R. solani, isolated from different families of plants.
Pathogenicity tests of isolated fungi on radish seeds
The pathogenicity of seven isolates of R.solani spp. (Al-Amiriya – Rs1, Al-Amiriya 2 – Rs2, Al-Khalidiya – Rs3, Sadat Al-fallujah – Rs4, Al-Nuaimiya – Rs5, Heet – Rs6 and Al-Saqlawiyah- Rs7) And seven isolates of Fusarium spp. (Al-Amiriya – Fu1, Al-Amiriya 2 – Fu2, Al-Khalidiya – Fu3, Al-Khalidiya – Fu4, Al-Nuaimiya – Fu5, Heet – Fu6Furthermore, Al-Saqlawiyah – Fu7) was tested. In the Department of Plant Protection at the College of Agriculture / University of Anbar, the test was carried out according to the method of 13 according to a Randomized completely design (CRD) sterile nutrient medium (Water Ager) was prepared and distributed in Petri dishes with a diameter of 9 cm containing15-20 mm. From the more significant culture medium and water (W.A.) (20 g acre, liter of distilled water), sterilized by an autoclaved 121 °C and a pressure of 5.1 bar/cm3 for 20 minutes, added to the antibiotic Amoxicillin at a concentration of 200 mg/L-1, the dishes were inoculated by taking a 5 mm diameter disc using A sterile cork piercing was placed in the middle of the dish. 3 dishes were used for each isolate of the tested fungi, and 3 were used as a comparison treatment (without adding a tablet from the mushroom colony). Then the dishes were transferred to the incubator and left at a temperature of 25 ± 2 for three days, after which the sterilized radish seeds were sown superficially with a solution of Sodium hypo chlori; 100%, 10 seeds were used for each plate. The seeds were sown in a circular motion around the fungal perennial disc so that the seeds were separated by 1 cm from the edge of the plate. Then, the plates were incubated for 14 days, after which the percentage of infection was calculated for each isolate according to the following equation:
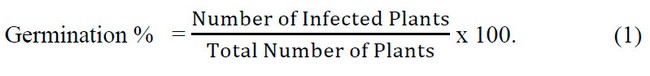
RESULTS AND DISCUSSION
The results of sampling in Fig. (1) and Fishow g. (1) Potato root rot disease is widespread worldwide in most potato growing areas, consistent with findings by 14, 15.

Figure 1: Potato plants infected with root rot disease.
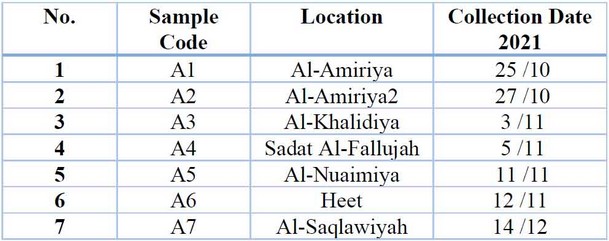
Table 1: Areas from which Potato root rot disease samples were collected.
Phenotypic diagnosis
The results of the phenotypic diagnosis shown in Table (2) indicated the presence of the fungus R.solani spp. and Fusarium spp., which were associated with potato root rot disease, which is the leading cause of the disease, and this is consistent with what was indicated by 16, 17.
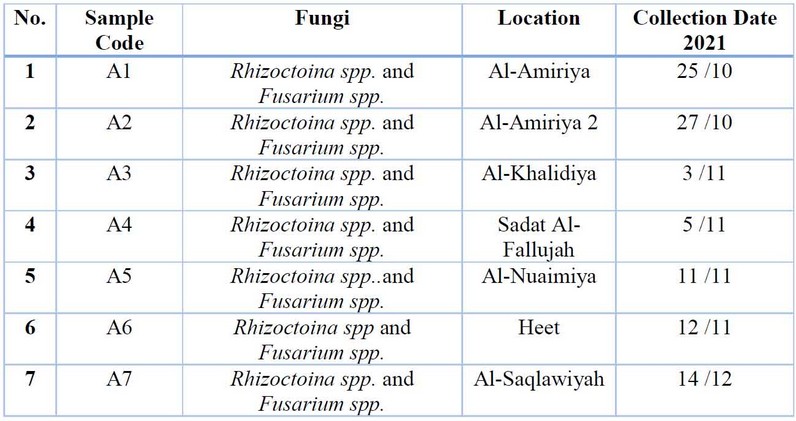
Table 2: The most essential fungi related to potato root rot were isolated and diagnosed phenotypically to the genus level.
Figure (2) The most important fungi that were phenotypically isolated and diagnosed
Molecular Diagnostics
Table (3) shows the results of the molecular diagnosis that the fungi that cause potato root rot disease are of the genus Rhizoctoniaspp. and Fusarium spp., according to the arrangement of nitrogenous bases in the single DNA strand. These results are consistent with what was mentioned by 16, 18, and 19, and these fungi are the leading cause of potato root rot disease.
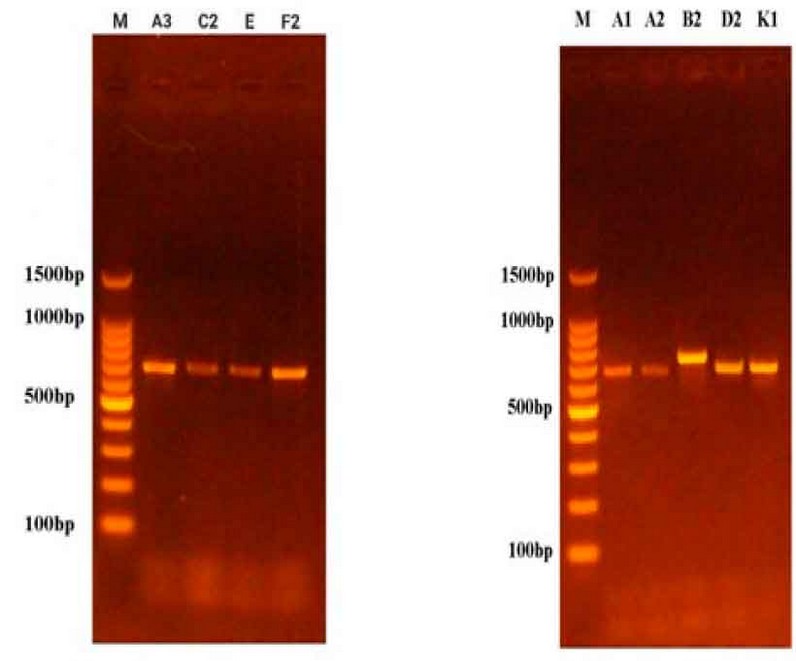
Figure 2: Results of ITS gene amplification of fungal species on Agarose Gel.
The pathogenicity test of some fungi isolates and their effect on the germination of radish seeds on the culture medium Water agar (W.A.)
Results showed in Table (4) that all tested fungi isolates tested on radish seeds were isolates of Rhizoctonia spp. and isolates ofFusarium spp. It significantly reduced the germination of radish seeds on water agar (W.A.) culture media compared to the control treatment that was not contaminated with any of the tested isolates Rhizoctonia, in which the infection rate was 0%. The test also showed the variation of the isolates in their effect on the germination of radish seeds, and the most influential of them was the isolate Rhizoctonia taken from Al-Anbar, Heet region, in which the infection rate was 90%. In contrast, Fusarium isolate is the strongest in the Al-Saqlawiya region, which achieved a 90% infection rate. Results agreed with that mentioned, and y 20, 21.
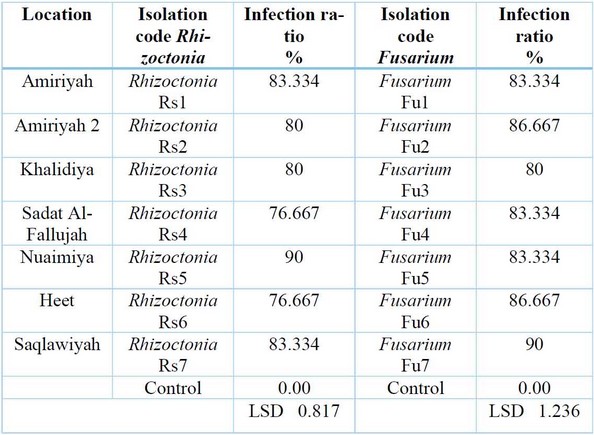
Table 4: Effects of different isolates of Rhizoctonia spp. and Fusarium spp. in germination of Radish seeds on water agar medium.
Table (4) Effects of different isolates of the mushroom Rhizoctonia spp.
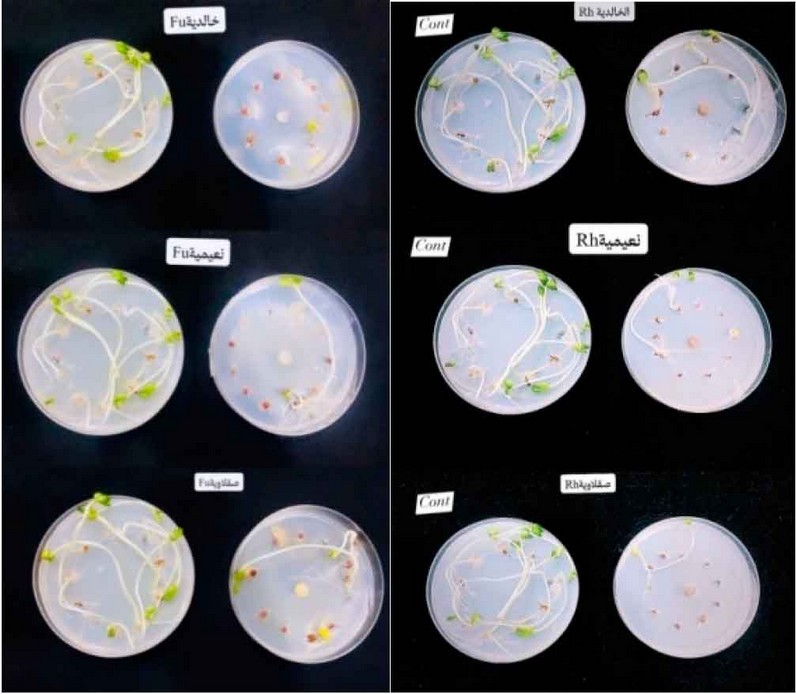
Figure 3: Fungus Rhizoctonia spp. and Fusarium spp. in germination of radish seeds on Water Agar media.
CONCLUSIONS
This study investigated the incidence and causative agents of potato root rot disease in Iraq. Through field sampling, phenotypic diagnosis, and molecular analysis, the research confirmed the widespread presence of the fungal pathogens Rhizoctonia spp. and Fusarium spp. These findings align with previous studies and highlight their role as the leading cause of this destructive disease in potato crops. Notably, the isolates demonstrated variable levels of pathogenicity against radish seeds, suggesting potential differences in virulence among strains. The research underscores the importance of implementing effective management strategies to combat potato root rot disease in Iraq and beyond.
REFERENCES
1. Tunio M.H., Jianmin G., Sher A. S., Imran A. L., Waqar A. Q., Kashif A. S., and F. A. Chandio. 2020. Potato production in aeroponics: An emerging food growing system in sustainable agriculture for food security. Chilean Journal of Agricultural Research. 80: (1).
2. Nasir, M.W. and Toth, Z.2022. Effect of Drought Stress on Potato Production: A Review. Agronomy, 12, 635. https://doi.org/ 10.3390/ agronomy 12030635.
3. Kamar U. , Sami u., Shafi M. , Shakil A. P. , Razia S. , Amit H. , Chao W. and C. H. Jian. 2021. In vitro and silico approach of fungal growth inhibition by Trichoderma asperellum HbGT6-07 derived volatile organic compounds. Arabian Journal of Chemistry. Volume 14, Issue 9.
4. Vilvert E., Stridh L. , Andersson B., Olson K., Aldén L. and A. Berlin. 2022. Evidence-based disease control methods in potato production: a systematic map protocol. Environmental Evidence .11:6. https://doi.org /10.1186/ s13750-022-00259-x .
5. Bodah E.T. 2017. Root Rot Diseases in Plants: A Review of Common Causal Agents and Management Strategies. Agri Res & Tech: Open Access J 5(3): ARTOAJ.MS.ID.555661.
6. Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J. 2016. FUN Guild is an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/ j. funeco. 2015.06.006.
7. Jiang, J., Yu, M., Hou, R., Li, L., Ren, X., Jiao, C. 2019. Changes in the soil microbial community are associated with Panaxquin quefolius L. root rot diseases. Plant Soil 438, 143–156. doi: 10.1007/s11104-018-03928-4.
8. Giachero, M. L.; Declerck, S.; Marquez, N. 2022. Phytophthora Root Rot: Importance of the Disease, Current and Novel Methods of Control. Agronomy, 12, 610. https:// doi.org/ 10.3390/ agronomy 12030610.
9. Agrios , G. N. 2005. Plant Pathology . 5th Ed. Elsevier Inc. USA. 998 pp.
10. Barnett, H. L., and B. B.Hunter. 1998. Illustrated genera of imperfect fungi. The American Phytopathological Society. U.S. Department of Agriculture, Agricultural Research Service, Washington State University, Pullman. APS Press. USA. St. Paul, Minnesota USA. 218p.
11. Lee, Y.M., Y.K. Choi and B.R. Min. 2000. Molecular characterization of Fusarium solani and its form aespeciales based on sequences analysis of the internal transcribed spacer (ITS) region of ribosomal DNA. Mycobiology. 28(2): 82-88.
12. Alhussaini M.S , Moslem M.A , Alghonaim M.I., Al-Ghanayem A.A. , Al-Yahya A.A.I ,HefnyH.M. and A. M. Saadabi. 2016. Characterization of Cladosporium Species by Internal Transcribed Spacer -PCR and Microsatellites-PCR.Pak. J. Biol Sci.; 19(4): 143-157. doi: 10.3923 /pjbs . 143.157.
13. Bolkan, H.H. and Butler, E.E. 1974. Studies on Hetero karyosis Virulence of Rhizoctonia solani. Phytopathology, 64: 513-522.
14. Vatankhah M. , Saberi -Riseh R. , Eskandari M. M. and H. A. fzali. 2019. Evaluation of some fungicides for the control of Fusarium dry rot of potato. J. Crop Prot. 2019, 8 (3): 275 -285 .
15. El-kazzaz, M.K.; Ghoneim, K.E.; Agha, M.K.M.; Helmy, A.; Behiry, S.I.; Abdelkhalek, A.; Saleem, M.H.; Al-Askar, A.A.; Arishi, A.A.; Elsharkawy, M.M. 2022. Suppression of Pepper Root Rot andWilt Diseases Caused by Rhizoctonia solani and Fusarium oxysporum. Life, 12, 587. https://doi.org/10.3390/ life12040587 .
16. Errampalli, D. and Johnston, H. W. 2001. Control of tuber -born black scurf (Rhizoctonia solani) and common scab (Streptomyces scabies) of potato with a combination of sodium hypochlorite and thiophanate -methyl preplanting seed tuber treatment. Canadian Journal of Plant Pathology, 23: 68 -77.
17. Sandipan, P. B., Solanki, B. P., Patel, N. N., Patel, R. L., Verma, P. D. and Desai, H. R. 2016. Effect of different fungicides against dry rot pathogens of potato caused by Fusarium spp. under in vitro condition. Cercetari Agronomic in Moldova, 4 (168): 69 -74.
18. Cullen D.W. , I. K. Toth, Y. Pitkin, N. Boonham, K. Walsh, I. Barker, and A. K. Lees. 2005. Use of Quantitative Molecular Diagnostic Assays to Investigate Fusarium Dry Rot in Potato Stocks and Soil. Phytopathology. DOI: 10.1094/ 95-1462·
19. Ghosh R. , Tarafdar A. and Sharma M. 2017. Rapid and sensitive diagnoses of dry root rot pathogen of chickpea (Rhizoctonia bataticola (Taub.) Butler using loop-mediated isothermal amplification assay. SCIenTIfICREPOrTS | 7:42737 | DOI: 10.1038/ srep42737 .
20. Majumdar, R.; Strausbaugh, C.A.; Galewski, P.J.; Minocha, R.; Rogers, C.W.2022. Cell-Wall-Degrading Enzymes-Related Genes Originating from Rhizoctonia solani Increase Sugar Beet Root Damage in the Presence of Leuconostocmes enteroides. Int. J. Mol. Sci. 2022, 23, 1366. https://doi.org/ 10.3390/ijms23031366.
21. Abo-Elyousr, K.A.M.; Ali, E.F.; Sallam, N.M.A.2022. Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropisprocera. Horticulturae , 8, 197. https:// doi.org/ 10.3390/ horticulturae. 8030197
22. Cullen, D.W., Toth, I.K., Pitkin, Y., Boonham, N., Walsh, K., Barker, I. and Lees, A.K.,. Use of quantitative molecular diagnostic assays to investigate Fusarium dry rot in potato stocks and soil. Phytopathology, 2005, 95(12), pp.1462-1471·
23. Ghosh, R., Tarafdar, A. and Sharma, M., Rapid and sensitive diagnoses of dry root rot pathogen of chickpea (Rhizoctonia bataticola (Taub.) Butler) using loop-mediated isothermal amplification assay. Scientific reports, 2017, 7(1), pp.1-12.
24. Ameen M. Shaman , Th. T. Mohammed. Effect Using Feed Additives Instead of Imported Premixes Affects the Physiology of Broiler Chickens. IOP Conference Series: Earth and Environmental Science.2023, 1262, 072080. https://doi.org/10.1088/1755-1315/1262/7/072080.
25. A. Jassim, A., I. Al-Jugifi, W. Gradually Increasing In Lighting Intensity On Characteristics Of Ross 308 Broiler Carcass And Some Internal Organs. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 205-213. doi: 10.32649/ajas.2023.179730.
26. Abo-Elyousr, K.A., Ali, E.F. and Sallam, N.M., Alternative Control of Tomato Wilt Using the Aqueous Extract of Calotropis procera. Horticulturae, 2022., 8(3), p.197..
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: A’shour, S.; Farhan, T. Identification or Diagnosis of Fungi that Cause Potato Root Rot. Revis Bionatura 2024; 9 (1) 51. http://dx.doi.org/10.21931/RB/2024.09.01.51
Additional information Correspondence should be addressed to saj20g7013@uoanbar.edu.iq.
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work usinghttps://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. First 13909355 Ecuador. Scopus coverage years: from 2016 to the present
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



















