Vol 9 No 1 2024-48
2024..09.01.48
Effect of Solanum aculeastrum on hematological parameters of Al-bino mice infected with Aspergillus fumigatus
Sara Ghalib Allwi Al-Saffy1 and Dalia Abdalkareem Abdulshaheed2*
1Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq; saragh199577@gmail.com.
2Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad. Iraq.
* Correspondence: dalia@covm. uobaghdad. Edu. I.Q.
Available from. http://dx.doi.org/10.21931/RB/2024.09.01.48
ABSTRACT
The goal of the current study was to research the changes in hematological parameters: WBC count, RBCs count, Hb, PCV, neutrophil, lymphocyte, and monocyte in albino mice infected with Aspergillus fumigatus by intraperitoneal injection after induced immunosuppression by intraperitoneal injection of cortisone. The current research also examined an attempt to reduce the infection load by treating Solanum aculeastrum. The result shows higher decreased significance (P≤0. 05) in RBCs, Hb, and PCV after being infected with A. fumigatus 7. 1 ± 0. 8, 11. 3 ± 0. 5 and 41. 5 ± 2. 4, respectively, while the total WBC count, neutrophil, lymphocyte, and monocytes were increased significantly (P≤0. 05) after treatment with S. aculeastrum in groups infected with A. fumigatus, compared to other groups. According to these results, we conclude that the alcoholic extract of S. Astrum has significant therapeutic and antifungal characteristics that lead to an increase in the total WBC count and, therefore, is considered a necessary alternative therapy for increasing immunity.
Keywords: Cortisone, Hematology, Fungi, Iraq.
INTRODUCTION
Fungi are a major, diverse, ubiquitous group of heterotrophic organisms that live as saprophytes or parasites or are related to many other organisms as symbiotes 1. According to estimates of global richness, it constitutes the second largest group of organisms, with about 3 million species expected. Also, it ranks third among the eukaryotic kingdoms regarding the wealth of known species 2, 3. The most prevalent pathogenic species in the animal kingdom is Aspergillus fumigatus, a saprotrophic fungus that lives vegetatively on decaying organic matter in the soil and spreads by asexual sporulation 4, 5. This fungus can survive high temperatures above (50°C) and may happen in piles of decomposing plant matter. The fungus releases a significant amount of asexual airborne spores 6. These fungi have a broad clinical spectrum, ranging from allergy to chronic infections and acute invasive aspergillosis (I.A.) in humans and animals 7. Aspergilli are known for their capacity to secrete different biologically active chemical compounds, including antibiotics, mycotoxins, immune-suppressant substances, and cholesterol-lowering factors 8. According to the host’s underlying immunological state, these fungi can cause overlapping chronic, noninvasive types of infection that range from the formation of a fungal ball (Aspergilloma) to a long-lasting inflammatory and fibrotic process that is presently categorized as chronic lung infection similar to invasive pulmonary aspergillosis (IPA) 4, 9.
Solanum aculeastrum, «goat bitter-apple» Hnzal (Arabic), is extensively dispersed in a native of Africa and South Africa. S. aculeastrum is a spiny perennial that grew to 3 m tall with white blooms and bears berries resembling lemons and turning yellow-green when ripe. For both people and domestic animals, the tart fruits of this plant are utilized medicinally in various techniques for the treatment of cancer, indigestion, and stomach disruption; the boiling extract of the fruits and leaves is administered orally route. Both fresh and cooked fruit are used as a therapy for acne, gonorrhea, and jigger wounds 10, according to the presence of bioavailable phyto-constituents including steroidal saponins, steroidal alkaloids, terpenes, flavonoids, lignans, sterols, phenolic compounds, and coumarins. Solanum spp. is essential in the nutraceutical and pharmaceutical industries. Both steroidal alkaloids and glycoalkaloids serve as important chemical indicators of this genus. Both ancient and modern systems of medicine place particular importance on steroid alkaloids and glycoalkaloids since they exhibit a variety of bioactivities, including antibacterial, analgesic, immunomodulatory, hepatoprotective, neurogenetic, anticancer, etc. 11. Considering the above facts and minimal studies, this study was designed to study the effects of S. aculeastrum on hematological parameters in albino mice infected with A. fumigatus.
MATERIALS AND METHODS
Fungal isolation
The fungus was isolated from different veterinary clinics and stray cats in Baghdad province, identified on Sabouraud Dextrose Agar and malt extract agar, and diagnosed by traditional morphological examination laboratory methods.
Preparation of A. fumigatus fungal suspension
The suspension of A. fumigatus was prepared according to a previously carried out study 12.
Solanum aculeastrum fruit extraction
Solanum aculeastrum (Hanzal) was purchased from local Baghdad markets, and the extraction was performed previously 13.
Induction of immunosuppression
Immunosuppression was induced by treatment of the mice with cortisone intraperitoneal administration at a single dose one day before conidial administration. Each mouse receives 2mg of Dexamethasone per mouse 14.
Animal and design experiments
Fifty-six albino mice were used in the study at 10-12 weeks and weighing 25. 3 ± 0. 9 g, maintained on a standard laboratory diet, water and temperature-controlled at the animal house laboratory at the College of Veterinary Medicine (University of Baghdad, Baghdad, Iraq)15, were randomly assigned to different groups: 8 mice per group as following:
· G1: Negative control mice (untreated).
· G2: Infected mice with a single dose of A. fumigatus at 2 × 107 cells/ml per mouse intraperitoneally (I/P).
· G3: Treated mice with cortisone at 2 mg/mice I/P.
· G4: Treated mice orally with a single dose of S. aculeastrum at 10 mg/kg B. W.
· G5: In which mice were treated with a single dose of cortisone at 2mg/mice I/P for one day before fungal spore infection and then infected with a single dose of A. fumigates 2 × 107 cells/ml per mouse I/P.
· G6: In which mice were treated with a single dose of S. aculestrum at 10 mg/kg B. W orally, one week after infection with a single dose of A. fumigates 2 × 107 cells/ml per mouse I/P.
· G7: Mice were treated orally with a single dose of S. aculestrum at 10 mg/kg B. W simultaneously with the fungal infection.
Blood samples collection
According to 16, the blood samples from all groups were taken at the end of the experiment.
Statistical analysis
SPSS calculated data for Windows TM version 23. 0 using one-way ANOVA. All experimental data are presented as Mean ± S.E. and significant differences at P≤ 0. 05 17.
RESULTS
Hematological study
Hematological parameters: total WBC counts, neutrophils, lymphocytes, monocytes, RBC count, Hb level, and PCV. The blood samples were taken from all the groups to analyze by employment tubes containing an anticoagulant agent in the laboratory. In the current study, the results showed that the control positive group of A. fumigatus infection, cortisone and group infected with A. fumigatus plus cortisone treated caused significant (p≤ 0. 05) decrease in RBCs count, Hb and PCV, whereas, no significant (p≤ 0. 05) differences were noticed in groups of mice treated with Solanumaculeastrum alone or plus cortisone and A. fumigatus in both G6 and G7 when compare with G1 (Table 1).
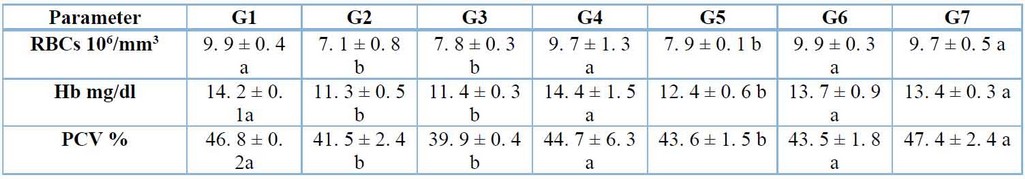
Table 1: Effects of A. fumigatus infection, cortisone, and S. aculeastrum treated after two weeks on parameters of RBC count, Hb, and PCV (mean ± S.E.).
Total WBCs, neutrophils, lymphocytes, and monocyte count were decreased significantly (p≤ 0. 05) in both groups infected with A. fumigates and S. aculeastrum alone. Also, mice representing control-positive cortisone decreased significantly (p≤ 0. 05) in neutrophils and monocytes. At the same time, total WBCs and lymphocytes were not affected significantly (p≤0. 05). The results also showed no significant differences (p≤0. 05) in a group of mice treated with cortisone plus infection of A. fumigatus. Total WBCs and lymphocytes were increased significantly (p≤0. 05), and neutrophils and monocytes were not affected significantly (p≤0. 05) in G6 treated with S. aculeastrum after one week of infection. Total WBCs, neutrophils, and lymphocytes were increased significantly (p≤0. 05), and monocytes were not affected significantly (p≤0. 05) in the group treated with S. aculeastrum at the same time of infection comparable with the controls regarding the WBCs (Table 2).
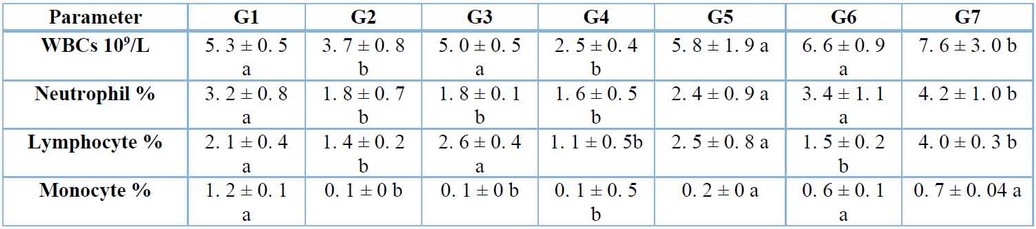
Table 2: Effects of Aspergillus fumigatus infection, cortisone, and Solanumaculeastrum treated after two weeks on parameters of (WBCs, neutrophils, lymphocytes, and monocytes) (mean ± S.E.).
DISCUSSION
The present results indicated that the hematological picture of A. fumigatus-infected mice is similar to that of another study 18. The mycotoxin from pathogenic fungi caused anemia, which was observed as decreases in PCV Hb% and RBC count. Also, the phospholipase enzyme in Aspergillus spp. is the essential virulence factor that can cause destabilization and penetration, break down membrane phospholipids surrounding the red blood cell, and generate arachidonic acid 19. Additionally, phospholipase hydrolyzes RBCs to release phosphatidylserine and produce lysophosphatidic acid (LPA), the latter of which results in the flow of substances through a blood cell membrane and causes swilling before exploding 20.
Previous studies also found decreased hematological parameters because of cortisone treatment 21, 22. Exposure to Dexamethasone demonstrates a significant change in red blood cells, such as anemia, destruction of cells, or inhibition of hematopoiesis. All these reasons affect RBC, Hb, and PCV levels, and these changes led to observing the result compared with a control group and explaining that Dexamethasone may cause suppression of the bone marrow. Such decreases could also be attributed to hemodilution. However, they could also result from RBC reaction or inhibition of RBC synthesis, which limits the capacity of these cells to absorb oxygen in conjunction with increased hemolysis brought on by excessive physical stress and results in severe anemia. The WBC decrease results in the lysis of neutrophils affected by phospholipase enzymes, the elimination of lipid phosphorus, and hydrolysis of membrane phospholipids occurred after exposure to this enzyme. This led to decreased cell size, sphering, and increased susceptibility to osmotic stress, which altered the cell’s functional properties and caused cell lysis 23. Another reason represented that phospholipase enzyme and Aspergillus spp. The infection effect on hormones responsible for the production of blood cells is represented by the erythropoietin hormone from the kidney, which is the primary catalyst for the production of blood cells; this finding agrees with other researchers 24.
The reduction in neutrophil and monocyte counts after cortisone-treated mice indicated immunosuppression. This finding agrees with another study 25. Steroids impair alveolar macrophage activity, lowering the primary defense against lung infection. Additionally, they affect T and B cell lymphocytes and reduce cytokine production, which impairs the adaptive immune response to invasive aspergillosis 26.
Solanum aculeastrum affects hematological parameters; the results indicated that plants cause a decrease in total WBC count and differential cells. WBC count is an indicator of an organism’s capacity to eradicate infection. A reduction in WBC count in the mice treated with Solanumaculeastrum group agrees with another study 27. The animal’s capacity to fight disease, attack, and destroy infectious agents in the blood may be adversely impacted by the decline in WBC levels. Additionally, the immune system’s effector cells may have a severe effect. Also, the WBC count decreases, as observed by other results 28. The decrease in WBC count suggests that the extract ingredients may have damaged or prevented the maturation of these blood cells. The committed stem cells that produce these blood cells are regulated by proliferation, differentiation, and maturation by granulocyte-macrophage colony-stimulating factors, interleukins IL-2, IL-4, and IL-5. Therefore, the extract might have interfered with the sensitivity of the committed stem cells responsible for generating these white blood cells and differential cells, or it might have decreased the synthesis of these regulatory factors 29-32.
Mice in both G6 and G7 showed an increase in total WBC count and its differential cells compared with other treated groups; best results were obtained when mice treated at the same time of infection compared with mice received the plant extract after one week of fungal spores infected, the stress factor of infection on the immune system and weakness of mice body can interfere with immune responses. Also, lymphocyte counts may be decreased with handling or other stressors and with age. Increased neutrophil counts (neutrophilia) are commonly seen in conditions of infection and acute inflammation and are related to immune system reactions to stress or excitement and infectious diseases 33.
Previous research proved that S. aculeastrum has a chemical and pharmaceutical component that acts as an antifungal against several fungal species 34, 35. Other researchers have investigated the effect of ethanolic extract using the diffusion method, and the findings showed that the most potent antifungal activity was by A. fumigates 13,36, 37. At the same time, other authors found that alcohol extracts from fruit ultimately impeded the growth of A. flavus (100%) 28, 35-37. The results of the present study agree with all the research above; the hematological findings showed improvements in white blood cells, neutrophils, lymphocytes and monocytes when given the ethanolic extract orally at the same time as infected with A. fumigatus, indicating the treatment power of the plant at the applied dose of 10 mg/kg B. W was elevated the immune status by increasing of immune cells that fight infection comparing with other groups38.
CONCLUSIONS
This study concluded that using the alcoholic extract of S. aculeasttrum could provide an effective therapeutic tool in the treatment of fungal infections and in correcting abnormalities in hematological and immunological markers due to exposure to different diseases. Furthermore, studies are of great importance to detect the efficacy of this extract on body organs and other infections.
Author Contributions: Conceptualization, S. G. A. A. and D. A. A.; methodology, S. G. A. A. and D. A. A.; software, S. G. A. A.; validation, S. G. A. A. and D. A. A.; formal analysis, S. G. A. A. and D. A. A.; investigation, S. G. A. A.; resources, S. G. A. A.; data curation, D. A. A.; writing-original draft preparation, S. G. A. A.; writing-eview and editing, S. G. A. A. and D. A. A.; visualization, D. A. A.; supervision, D. A. A.; project administration, S. G. A. A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Scientific Ethics Committee of the Department of Microbiology at the College of Veterinary Medicine (University of Baghdad, Baghdad, Iraq).
Acknowledgments: The authors dramatically acknowledge the Head Department and the staff of the Department of Microbiology at the College of Veterinary Medicine for their valuable support.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. McCoy, C.W; Samson, R.A; Boucias, D.G. Entomogenous fungi.Handbook of natural pesticides, CRC Press, 2019, pp.151-236.
2. Barkai-Golan, R.; Paster, N. Mycotoxins in fruits and vegetables.Elsevier, 2011, pp. 74.
3. Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev 2019, 33(1), 140-148.
4. Tell, L.A.; Burco, J.D.; Woods, L.; Clemons, K.V. Aspergillosis in birds and mammals: considerations for veterinary medicine. In Recent Developments in Fungal Diseases of Laboratory Animals. Springer, Cham. 2019. pp.49-72.
5. Schoustra, S.E.; Debets, A.J.; Rijs, A.J.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Verweij, P.E. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, the Netherlands. Emerg Infect Dis 2019, 25 (7), 1347-1353.
6. Resendiz Sharpe, A.; Lagrou, K.; Meis, J.F.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E.; ISHAM/ECMM Aspergillus Resistance Surveillance Working Group. Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol 2018, 56(suppl_1), S83-S92.
7. May, G.S. Mitogen-activated protein kinase pathways in Aspergilli.In The aspergilli.CRC Press. 2007, pp.141-148.
8. Dewi, I.M.; Janssen, N.A.; Rosati, D.; Bruno, M.; Netea, M.G.; Brüggemann, R.J.; van de Veerdonk, F.L. Invasive pulmonary aspergillosis associated with viral pneumonitis.Curr Opin Microbiol 2021, 62, 21-27.
9. Koduru, S.; Grierson, D.S.; Afolayan, A.J. Antimicrobial Activity of Solanumaculeastrum. Pharm Biol 2006, 44(4), 283-286.
10. Patel, P.; Prasad, A.; Srivastava, K.; Singh, S.S.; Chakrabarty, D.; Misra, P. Updates on steroidal alkaloids and glycoalkaloids in Solanum spp.: Biosynthesis, in vitro production and pharmacological values. Stud Nat Prod Chem 2021, 69, 99-127.
11. Su, H.; Li, C.; Wang, Y.; Li, Y.; Dong, L.; Li, L.; Zhu, M. Kinetic host defense of the mice infected with Aspergillus Fumigatus. Future Microbiol 2019, 14(8), 705-716.
12. Eidi, S.; Azadi, H.G.; Rahbar, N.; Mehmannavaz, H.R. Evaluation of antifungal activity of hydroalcoholic extracts of Citrulluscolocynthis fruit. J Herb Med 2015, 5 (1), 36-40.
13. Marina A.Shevchenko, Andrey O.Bogorodskiy, Natalia I.Troyanova, Ekaterina A.Servuli, Elena L.Bolkhovitina, Georg Büldt, ChristophFahlke, Valentin I. Gordeliy, Thomas Gensch, Valentin I. Borshchevskiy, Alexander M. Sapozhnikov, «Aspergillus fumigatus Infection-Induced Neutrophil Recruitment and Location in the Conducting Airway of Immunocompetent, Neutropenic, and Immunosuppressed Mice. J Immunol Res 2018, 12, 20-28.
14. Gargiulo, S.; Greco, A.; Gramanzini, M.; Esposito, S.; Affuso, A.; Brunetti, A.; Vesce, G. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J 2012, 53(1), E55-E69.
15. Coles, E.H. Veterinary clinical Pathology 4th ed WB Saunders company Philadelphia.London, Toronto, Mexico, Riodejenario, Sydney, Tokyo Hong Kong, 1986, 136-170.
16. Gharban, H.A. Cumulative Effect of Subclinical Mastitis on Immunological and Biochemical Parameters in Cow Milk.Arch Razi Instit, 2021, 76 (6), 1599-1608.
17. Mohamad, S.H.; Thalij, K.M.; AL-Bander, K.; Dheeb, B.I. Effects of Allergic fungi on hematological and immunological parameters of human patients and rabbits. Egypt Acad J Biol Sci GMicrobiol 2014, 6(2), 41-48.
18. Mansoor, S. S.; Al-Esawi, J. S. . .; Al-Falahi, M. N. Assessing The Efficiency Of Cement Kiln Dust For Heavy Metals Removal From Simulated Polluted Water. JLSAR 2023, 4, 45-52.
19. Neidlinger, N.A.; Larkin, S.K.; Bhagat, A.; Victorino, G.P.; Kuypers, F.A. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem 2006, 281 (2), 775-781.
20. Razzaq, S.A.; Jaber, I.J.; Kadhim, S.A.; Abbas, A.A. Pharmacological Effects of Dexamethasone in Rats. Indian J Forensic Med Toxicol 2020,14 (3), 1003.
21. Ribas, J.L.C.; Zampronio, A.R.; Silva De Assis, H.C. Effects of trophic exposure to diclofenac and Dexamethasone on hematological parameters and immune response in freshwater fish. Environ Toxicol Chem 2016, 35(4), 975-982.
22. Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 2000,13 (1), 122-143.
23. Auda, M.A. Effect of phospholipase and the fungus which it produced (Aspergillusniger) on the some of blood parameters of male mice (Musmusculus). Al-Qadisiyah J Pure Sci 2009, 14 (1). 22-39.
24. Al-Maliki, S.J.; Al-Ali, A.A.; Kathim, A.S. Effect of corticosteroids cortisol hormone [hydrocortisone] on the of the blood parameter in pregnant and non-pregnant laboratory females mice. J Histol Cell Biol 2018, 1 (1): 16-22.
25. S. Hameed, T., Sawicka, B. Role Of Agricultural Extension In Adoption Of Sustainable Agriculture Practices. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 250-260. doi: 10.32649/ajas.2023.179947.
26. A A Al-Azzami , Th T Mohammed . Effect of Adding Dry Leaves of Lemongrass (Cymbopogon Citratus) To the Diet on Some Biochemical Tests of Blood in Broiler (Ross 308). IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12125. https://doi.org/10.1088/1755-1315/1252/1/012125.
27. Aboyade, O.M.; Yakubu, M.T.; Grierson, D.S.; Afolayan, A.J. Studies on the toxicological effect of the aqueous extract of the fresh, dried and boiled berries of SolanumaculeastrumDunal in male Wistar rats.Hum Exp Toxicol 2009, 28 (12), 765-775.
28. Aboyade, O.M.; Yakubu, M.T.; Grierson, D.S.; Afolayan, A.J. Safety evaluation of aqueous extract of unripe berries of Solanumaculeastrum in male wistar rats. Afr J Pharm Pharmacol 2010, 4(3), 90-97.
29. Al-gharban, H.A.; Dhahir, S.H. Serological diagnosis of persistent infection with Anaplasma marginale bacteria in cattle.Iraqi J Vet Med 2015, 39 (1), 33-39
30. Gharban, H.A.; Yousif, A.A. Serological and Molecular Phylogenetic Detection of Coxiella burnetii in Lactating Cows, Iraq.Iraqi J Vet Med 2020, 44(E0), 42-50
31. Gharban, H.A.; Yousif, A.A. Serological, Clinical and Hematological prevalence of Coxiella burnetii in Adult Cows, Iraq. Biochem Cell Arch 2020, 20(1), 67-74.
32. Ameen M. Shaman , Th. T. Mohammed. Effect Using Feed Additives Instead of Imported Premixes Affects the Physiology of Broiler Chickens. IOP Conf Ser Earth Environ Sci 2023, 1262 (7), 72080. https://doi.org/10.1088/1755-1315/1262/7/072080.
33. A A Al-Azzami , Th T Mohammed . The Effect of Adding Lemongrass Leaf Powder (Cymbopogon Citratus) to the Diet as a Natural Supplement on Some Productive Traits and Oxidation Indicators in Broiler (Ross 308). IOP Conf Ser Earth Environ Sci 2023, 1252 (1), 12123. https://doi.org/10.1088/1755-1315/1252/1/012123.
34. O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Brown, D.E. Practical murine hematopathology: a comparative review and implications for research. Comp Med 2015, 65(2), 96-113. .
35. Gurudeeban, S.; Ramanathan, T.; Satyavani, K.; Dhinesh, T. Antimicrobial effect of coastal medicinal plant †«Citrulluscolocynthis against pathogenic microorganisms. Afr J Pure Appl Chem 2011, 5(5), 119-122.
36. Hameed, B.; Ali, Q.; Hafeez, M.M.; Malik, A. Antibacterial and antifungal activity of fruit, seed and root extracts of Citrulluscolocynthis plant. Biol Clin Sci Res J 2020, 19 (1), 1-15.
37. Mohammad, H.A.; Ajaj, E.A.; Gharban, H.A. The first study on confirmation and risk factors of acute and chronic canine distemper in stray dogs in Wasit Province, Iraq, using enzyme-linked immunosorbent assay and reverse transcription-polymerase chain reaction. Vet World 2022, 15 (4), 968-974
38. Koduru, S.; Grierson, D.S.; Afolayan, A.J. Antimicrobial Activity of Solanumaculeastrum.Pharm Biol 2006, 44(4), 283-286.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Chango, M.; Rosero, G.; Erazo, N.; Álvarez, P. Effect of Solanum aculeastrum on hematological parameters of Albino mice infected with Aspergillus fumigatus. Revis Bionatura 2024; 9 (1) 48. http://dx.doi.org/10.21931/RB/2024.09.01.48
Additional information. Correspondence should be addressed to dalia@covm.
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. First 13909355 Ecuador. Scopus coverage years: from 2016 to the present
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



















