Vol 9 No 1 2024-16
2024..09.01.16
Somatic Cell Count Evaluation in Early Lactation between Primiparous and Multiparous Bos indicus Cows
Santiago Alexander Guamán-Rivera1,*, Angela Edith Guerrero-Pincay1, Nelson Rene Ortiz-Naveda2, Raúl Lorenzo González-Marcillo1 and Robinson J. Herrera-Feijoo3
1 Escuela Superior Politécnica de Chimborazo (ESPOCH), Sede Orellana, El Coca 220150,
Ecuador; a_guerrero@espoch.edu.ec
raul.gonzales@espoch.edu.ec
2 Facultad de Ciencias de la Tierra, Universidad Estatal Amazónica, Puyo, Pastaza, Ecuador
nr.ortizn@uea.edu.ec
3 Universidad Técnica Estatal de Quevedo, Quevedo 120550, Ecuador; rherreraf2@uteq.edu.ec
* Correspondence: santiagoa.guaman@espoch.edu.ec
Available from. http://dx.doi.org/10.21931/RB/2024.09.01.16
ABSTRACT
Using Bos indicus cows, a study examined the relationship between somatic cell count (SCC) and milk yield. For this study, one hundred fifty cows (Primiparous, PM, 75 and Multiparous, MP 75) in early lactation (days in milk, PM =134 ± 3; MP = 136 ± 5), milk production (9,88 kg/d, on average) of the creole breed Gyr lechers were enrolled. Before being assigned to each treatment, the SCC values were lower than 220,000 cells/mL, on average. All cows were maintained to graze daily on Megathyrsus maximus and supplemented with Morus alba ad libitum, being hand-milking at 0700 daily. Before analysis, the SCC was logarithmically transformed (log10). Then, PROC Mixed from SAS version 9.4 was used to evaluate all measurements. Regarding our results, the MP had greater milk yields than PM cows (10.83 vs. 9.18 ± 0.38 kg/d; P = 0.003). Similar results were observed for fat-corrected milk (8.26 vs. 6.80 ± 0.34; P = 0.002), although the fat values did not differ between both groups (P = 0.86) being lower than referential values for these breeds (2.46 ± 0.16, on average). No differences were observed in the other milk components (P = 0.65 to 0.85). Despite that, the somatic cell count (SCC) values showed a statistical tendency in PM than in MP (1.89 vs. 2.13 ± 0.05; P= 0.07). In conclusion, low-fat contents were observed in both groups, possibly due to the low quality of foods used in ruminant feeding. While that, the parity and advanced lactation conditioned the SCC contents. Therefore, other studies should be performed to identify more factors that could be determinants.
Keywords: Milk, Tropical livestock, Udder health
INTRODUCTION
According to Britt et al. 1, by 2067, the world’s population is predicted to reach 10.4 billion, reducing the arable land available for food production. So, the sustainability of dairy farms will be vital to stopping the growing agricultural frontier. Compared to breeds from temperate regions, tropical bovine production is low regarding milk kilos, composition, or udder health. As a result, developing and tropical nations continue to face difficulties in increasing milk output2,3. Nevertheless, mastitis is among the costliest diseases affecting the dairy cattle industry4. Most of the immune cells in milk are lymphocytes, polymorphonuclear neutrophils (PMN), and macrophages, represented by the somatic cell count (SCC)5. This udder inflammation can be subclinical and chronic, but both forms have severe risks to obtaining milk of good hygienic quality and farming profitability6. In addition, the resilience of high-yielding animals and poor efficacy of therapies and prevention (i.e., antibiotic resistance and dubious efficacy of vaccines) could be associated factors. In this sense, one of the most used indicators to assess milk quality and define milk prices is SCC8. According to Juárez et al.6, the first and principal tool used by technicians and farmers to evaluate udder health in flocks is SCC, an essential tool with easy application. Although much scientific evidence has been reported from other continents9–11 using breeds of Bos taurus at the Latin American level, few studies where the raising Bos indicus predominates have been informed. Data from MAGAP12 have shown that in the Amazon region, cattle raising represents one of the more important activities to generate money resources for these families2. In fact, of 5236 agricultural productive units (UPA’s) identified in Orellana province, approximately 46% are developed as small livestock farmers with low technological levels and bad management of their biotic resources2,13–15. Therefore, in this scenario, no studies have been conducted to explore the udder’s health status and can recommend some mastitis control practices. Aimed this, this first study carried out in Orellana province aimed to explore the udder health in early lactation in Bos indicus cows.
MATERIALS AND METHODS
Dairy Farms
This study collected milk samples from one livestock farm in Joya de los Sachas, Orellana, Ecuador. According to González Marcillo et al.13, the humid tropical rainforest conditions characterize this area’s climate. Its altitude is 275 m above sea level, and the average annual rainfall is 2942 mm. The average yearly temperature is 29.7 °C. One hundred fifty cows (Primiparous, PM, 75 and Multiparous, MP 75) in early lactation (days in milk, PM =134 ± 3; MP = 136 ± 5), milk production (9,88 kg/d, on average) of the creole breed Gyr lechers were enrolled. The cows were maintained to graze daily on (Megathyrsus maximus, CP 12%) and supplemented with white mulberry (Morus alba) ad libitum, being all cows every day hand-milked at 0700. Before being assigned to each treatment, the SCC values were lower than 220,000 cells/mL, on average.
Milk samples
A complete design block with a randomized PM and MP was used to disperse the cows. The investigation lasted for 21 days. As a result, 50 mL samples of milk were aseptically collected, conserved with antimicrobial tablets (Bronopol, Broad Spectrum Micro-tabs II, D&F Control Systems Inc., San Ramon, CA), and maintained at 4°C until processing for milk composition and SCC analyses. Then, in the Uyunbicho at the Central University of Ecuador, milk composition was examined using infrared absorption, and SCC was calculated using an automatic somatic cell counter that had previously been calibrated for cow milk (Fossomatic 500, Foss-Electric, Hillerd, Denmark).
Statistic evaluation
PROC Mixed from SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used to analyze the data. The SCC was logarithmically converted (log10) prior to analysis. While residual error and cows were regarded as random variables, our fixed effects (PM and MP) were included in the model. With SAS’s PDIFF option, differences between least squares means were calculated, and the Tukey test was used to compare them. Additionally, the PRO CORR of SAS was associated with the milk composition and SCC. Statistical differences and trends were announced at P ≤ 0.05 and P ≤ 0.10, respectively.
RESULTS
Table 1 shows the milk composition and SCC compared to primiparous (PM) and multiparous (MP).
Days in milk were estimated to be 134 and 136 days for primiparous and multiparous does, respectively, at the start of the trial (P > 0.05). Comparing primiparous and multiparous women’s milk yields revealed differences (P = 0.003); the MP showed a 15% higher milk yield than those in PM (10.83 vs. 9.18 ± 0.38 kg/d; Table 1). As for FCM values, although the fat contents did not change between treatments (2.46 ± 0.16%, on average; P = 0.68), the corrected milk by fat was greater in MP than those obtained for PM (8.26 vs. 6.80 ± 0.34%; Table 1)
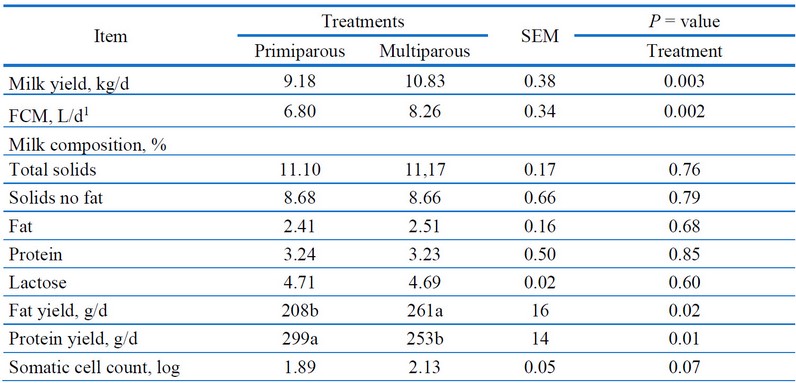
1Fat corrected milk at 4%; FCM = kg of milk yield × [0.4+ 0.15 × (fat %)].
Table 1. Comparisons between the somatic cell counts (SCC) and milk yield features of 120 primiparous and multiparous Bos indicus cows.
In contrast, the other milk components did not differ between treatments (P = 0.60 to 0.85%; Table 1). Overall, the mean values were for total solids (11.14 ± 0.17%), solids non-fat (8.67 ± 0.66%), protein (3.24 ± 0.50%) and lactose (4.7 ± 0.02%). Nevertheless, when comparing the fat and protein contents expressed as g/d, the MP had greater fat contents than PM cows (261 vs. 208 ± 16; P 0.02) but with lower protein contents (253 vs. 299 ± 14; P 0.01; Table 1). On the other hand, statistical tendencies were observed for SCC (P = 0.07). In the current study, the overall averages of SCC were 153.703 × 103 mL-1 (log SCC 1.89) and 394.560 × 103 mL-1 (log SCC 2.13) for primiparous and multiparous cows, respectively. As shown in Figure 1, the regression analysis showed strong significant associations between total solids and fat (P < 0.001) and solids non-fat and CP (P < 0.001), as well as for SCC and lactose contents (P < 0.001).
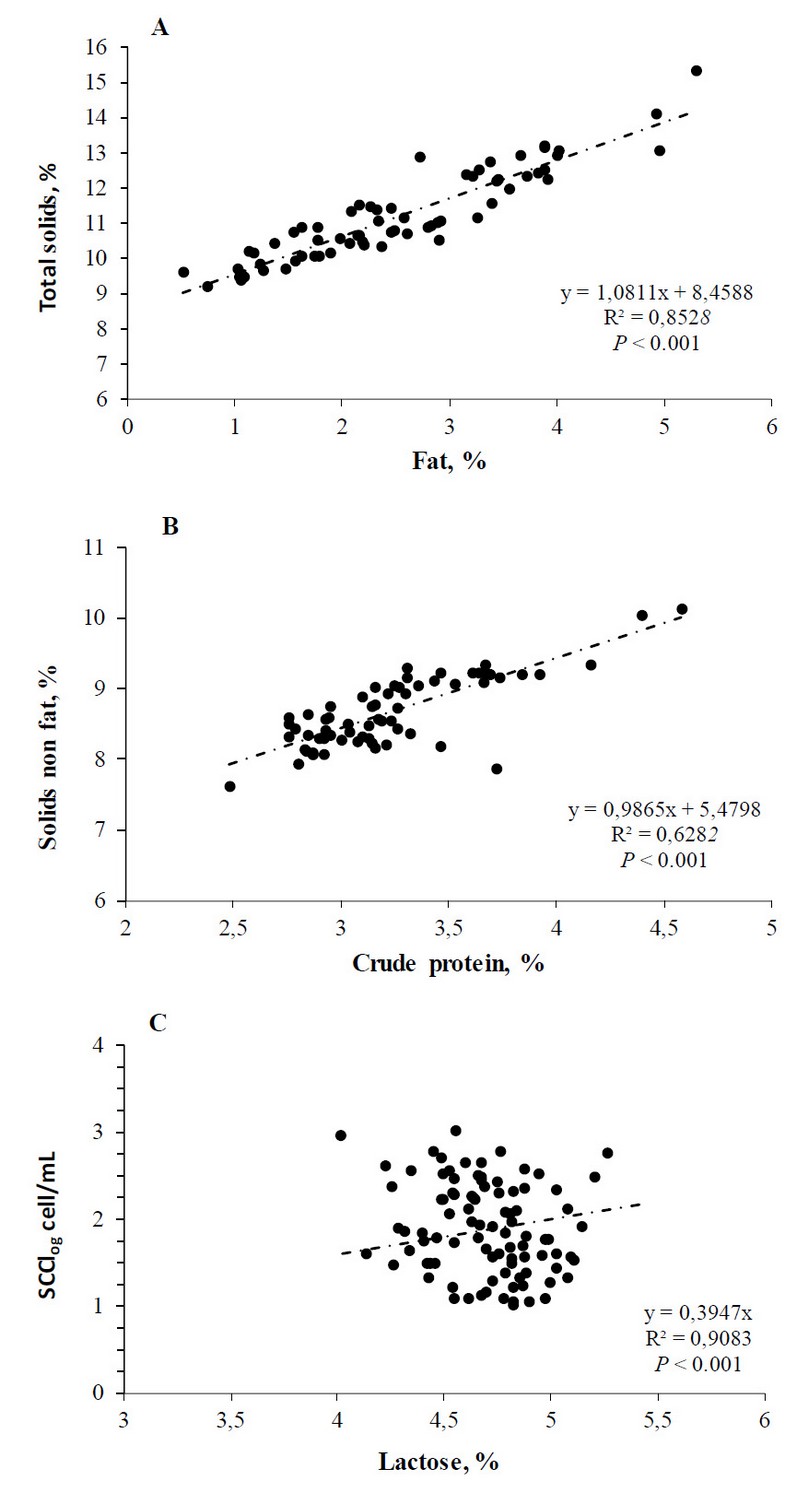
Figure 1. Regression analysis is linear for total solids and fat (A), solids non-fat and CP (B) and SCC and lactose (C).
Table 2 displays the simple correlation coefficients for milk yields, composition, and SCC. This study did not link Milk yield and SCC (-0.04 to 0.67; P = 0.50 to 0.64). Nevertheless, there were significant correlation coefficients for PM and MP between total solids and fat (r = 0.92 to 0.94; P < 0.001) and solids non-fat and CP (r = 0.79 to 0.90; P < 0.001: Table 2).
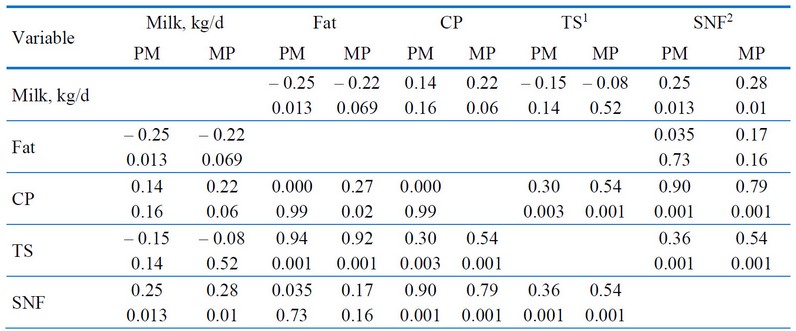
1 TS, total solids; 2SNF, Solids non-fat; PM, primiparous cows and MP, multiparous cows.
Table 2. Somatic cell counts of primiparous (PM) and multiparous (MP) Bos indicus cows were correlated simply with milk yield attributes (n = 120).
DISCUSSION
Coulibaly and Nialibouly16 observed that the evaluation of milk yield of lactating cows in tropical conditions is confounded by the fact that the calf must initiate milk letdown. Martínez-Velázquez et al.17, using creole, Gujarat have reported at 210 DIM a higher milk yield (22 ± 1.7 kg/d) and fat (2.81 ± 0.1%) than those obtained in our study. Millogo et al.3 in zebu dairy cattle at 41 ± 6 DIM observed lower milk yields than this study (1.3 vs. 10 kg/d, on average) but with more excellent fat contents (4.88 vs. 2.46 %). We hypothesized that our lower fat contents observer in this study could be related to poor forage quality for bad grazing management practices, as evidenced by Guaman-Rivera et al.2,18 in Orellana province. According to Bonfoh et al.19 and Farahani, Amanlou, and Kazemi-Bonchenari 20, the fat content in milk reflects supplementary feeding for the entire dry period, which in our conditions was not done.
However, it’s crucial to remember that the variation in the amount of milk the calf suckled greatly impacted the daily variance in saleable milk yield. Additionally, since milk fat content rises during udder emptying, variation in the degree of udder evacuation and calf suckling may impact the fat content3. Another critical point to consider in this study is that the fat contents did not vary between PM and MP, but Bonfoh et al.20 reported a higher fat content of Malian Zebu milk when the milk yield decreased. Based on the abovementioned evidence, estimating milk production in lactating zebu cows has always been difficult since using their calves to stimulate milk letdowns16 is necessary. Our research team decided to do this work at 134 DIM, avoiding the suckled effect.
As expected, the lactose contents did not differ between PM vs. MP (4.70 ± 0.02% on average), being their values like those reported by Millogo et al.19 (4.84%), Martínez-Velázquez et al.17 (4.71%), Sidibe-Anago, Ouedraogo, and Ledin21 (4.6%). Despite weak correlations in PM and MP cows between SCC and lactose contents (r = 0.22 to 0.25), it was significant (P = 0.03 to 0.04).
According to Portnoy and Barbano22, lactose is the main carbohydrate in milk at a concentration of around 4.6% on an anhydrous basis. Consequently, it is a key component in milk synthesis and secretion, regulating the osmotic equilibrium in the mammary cell. Decreased milk yield and compositional changes in milk, particularly concerning lactose concentration, have been reported in infected mammary glands in cows23–25. High-quality milk production is a primary factor for the safety and quality of dairy products26. Mastitis is a significant problem in dairy farming globally5, occasioning also considerable economic losses. Besides this, Kirkeby et al.5 stated that mastitis is a predominant reason for antibiotic use in dairy products and can impair animal welfare. The SCC in milk indicates the inflammatory response in the mammary gland27. In cattle breeds of origin Bos taurus, ample scientific evidence stated that the optimal cut-off points to distinguish between infected and uninfected quarters should have less than 200,000 cells/mL28,29. However, in Bos indicus cattle breeds, information is scarce and confused on referential SCC values to consider as uninfected. At the level of Ecuador, legal normative declared by INEN30 is regarded as an uninfected quarter when the SCC values are less than 500,00 cells/mL. Bonfoh et al.19 reported that 9% of cows had a mean value of SCC greater than 654,000 cells/mL. Similar results have been observed by Juozaitiene, Juozaitis, and Micikeviciene31 (SCC, 800,000 cells/mL). In this first study, in PM cows (n = 75), 57 and 43% had lower SCC values than 39,000 and 180,000 cells/mL, respectively.
Meanwhile, for MP cows (n = 75), the SCC values were 65% (> 81,000 cells/mL) and 35% (>979,000 cells/mL). Based on these findings, the MP cows showed to have more risk of obtaining subclinical mastitis, according to Juozaitiene et al.31. In SCC values of dairy ewes, Orman et al.31 reported a negative correlation between milk yield and parity. This supported our results, in which the PM cows showed lower SCC values when compared to those of MP cows. In these tropical conditions, due to high ambient temperatures with high relative humidity. The cattle could face heat stress through physical, biochemical, and biological changes resulting in decreased production performance and poorer immunity (i.e., a high SCC), such as observed 32. Although most milk parameters show substantial mastitis-related changes, no correlations were found between SCC and all milk components in this study. Nevertheless, we did not discard possible seasonal effects, stage of lactation, genetics (breed), productive systems and udder and teat morphology-like associated factors in Bos indicus cows33.
CONCLUSIONS
Based on these findings, the lower fat contents observed in PM and MP cows could be related to forages of poor nutritional quality. In addition, the present investigation evidenced a progressive increase in SCC with parity and advanced lactation. So, this first study performed in Orellana Province might be a point to start formulating feeding strategies for achieving greater and lower milk composition and SCC, respectively.
Author Contributions: Conceptualization, SAGR and RJHF; methodology, AEGP, NRON and RLGM; software, SAGR and AEGP; validation, SAGR and RJHF; formal analysis, AEGP, NRON and RLGM; investigation, SAGR and RJHF; resources, AEGP, NRON, SAGR, RJHF and RLGM; data curation, SAGR and AEGP; writing—original draft preparation, AEGP, NRON, SAGR, RJHF and RLGM; writing—review and editing, SAGR and RJHF; visualization, AEGP and RLGM; supervision, SAGR and RJHF; project administration, RLGM; funding acquisition, AEGP, NRON, SAGR, RJHF and RLGM All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Acknowledgments: We thank the project Silvopastoral system in Orellana Province. In addition, we thank Theofilos Toulkeridis for the grammar check of this manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Britt JH, Cushman RA, Dechow CD, Dobson H, Humblot P, Hutjens MF, et al. Invited review: Learning from the future—A vision for dairy farms and cows in 2067. J Dairy Sci. 2018;101:3722-3741. http://dx.doi.org/10.3168/jds.2017-14025.
2. Guaman-Rivera S., Guerrero-Pincay A, Ortiz-Naveda N., González-Marcillo R. Prediction of the nutritional values by INRA (2018) feed evaluation system of Megathyrsus maximus subjected to different grazing strategies. J Agric Environ Int Dev. 2023;117: 117-140.
3. Millogo V, Ouédraogo GA, Agenäs S, Svennersten-Sjaunja K. Day-to-day variation in yield, composition and somatic cell count of saleable milk in hand-milked zebu dairy cattle. African J Agric Res. 2009;4:151-155.
4. Damm M, Holm C, Blaabjerg M, Bro-Novak M, Schwarz D. Differential somatic cell count—A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. Minerva Med. 2017;60:3922-3927. http://dx.doi.org/10.3168/jds.2016-12409.
5. Kirkeby C, Toft N, Schwarz D, Farre M, Nielsen SS, Zervens L, et al. Differential somatic cell count as an additional indicator for intramammary infections in dairy cows. J Dairy Sci. 2020;103:1759-1775. http://dx.doi.org/10.3168/jds.2019-16523.
6. Juárez M., Blanco M., De La Fuente L., Beneitez E, Gonzalo C, Carriedo J., et al. Factors of Variation Influencing Bulk Tank Somatic Cell Count in Dairy Sheep. J Dairy Sci. 2010;88(3):969–74.
7. Riva F, Latorre A., Moroni P. Ruminant mastitis: A 360° view. Vol. 9, Frontiers in Veterinary Science. 2022.
8. Raynal-Ljutovac K, Pirisi A, de Crémoux R, Gonzalo C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin Res. 2007;68: 126-144.
9. Shangraw EM, McFadden TB. Graduate Student Literature Review : Systemic mediators of inflammation during mastitis and the search for mechanisms underlying impaired lactation. J Dairy Sci. 2022;105,20776. http://dx.doi.org/10.3168/jds.2021-20776.
10. Shangraw EM, Rodrigues RO, Mcfadden TB. Intramammary lipopolysaccharide infusion induces local and systemic effects on milk components in lactating bovine mammary glands. J Dairy Sci. 2020; 103(8), 7487-7497. http://dx.doi.org/10.3168/jds.2019-18022.
11. Shangraw EM, Rodrigues RO, Witzke MC, Choudhary RK, Zhao F-Q, Mcfadden TB. Intramammary lipopolysaccharide infusion induces local and systemic effects on milk components in lactating bovine mammary glands. J Dairy Sci. 2019;103:7487-7497. http://dx.doi.org/10.3168/jds.2019-18022.
12. MAGAP. Agricultural stadistic. Available online: www-magap.gob.ec. Accessed on 14 January 2022.
13. González Marcillo RL, Castro Guamàn WE, Guerrero Pincay AE, Vera Zambrano PA, Ortiz Naveda NR, Guamàn Rivera SA. Assessment of Guinea Grass Panicum maximum under Silvopastoral Systems in Combination with Two Management Systems in Orellana Province, Ecuador. Agriculture. 2021;11(2):117.
14. Fuentes O., Guamán S., Zacarías F, Paredes V. Silvopastoral Systems as a Strategy for Reconversion of Livestock Farming in Ecuadorian Amazon. Adv Compos Bull. 2023;1:135-138.
15. Guamán Rivera SA., Marcillo González RL, Carrasco R, Guamán Quinche F. Caracterización de los Sistemas Ganaderos de Aptitud Lechera en el Valle del Quijos, Provincia del Napo, Ecuador. Eur Sci J ESJ. 2019;31;(15):279–792. http://eujournal.org/index.php/esj/article/view/12082/11516.
16. Coulibaly M, Nialibouly O. Effect of suckling regime on calf growth, milk production and offtake of zebu cattle in Mali. Trop Anim Health Prod.1998;30:179-189.
17. Martínez-Velázquez G, Palacios-Fránquez J., Bustamante-Guerrero J., Ríos- Utrerab A, Vega-Murillo V., Montańo-Bermúdez M. Composición de leche de vacas Criollo, Guzerat y sus cruzas F1 y su relación con el peso al destete de las crías. Rev Mex Ciencias Pecu. 2010;1:311-324.
18. Guamán-Rivera S., Mira-Naranjo M., Peralta-Paredes VA, Aragón-vásquez E. Animal Diseases Reported by Livestock Farmers in Orellana Province , Ecuador : A Retrospective Observational Study from 2011 to 2019. Seybold Rep J. 2023;18:7-24.
19. Bonfoh B, Zinsstag J, Farah Z, Simbé C., Alfaroukh I., Aebi R, et al. Raw milk composition of Malian Zebu cows (Bos indicus) raised under traditional system. J Food Compos Anal. 2005;(1):18:29-38.
20. Farahani TA, Amanlou H, Kazemi-Bonchenari M. Effects of shortening the close-up period length coupled with increased supply of metabolizable protein on performance and metabolic status of multiparous Holstein cows. J Dairy Sci. 2017;100:6199-6217. http://dx.doi.org/10.3168/jds.2016-12263.
21. Sidibe-Anago AG, Ouedraogo GA, Ledin I. Effect of partly replacing cottonseed cake with Mucuna spp. (var. Ghana) hay on feed intake and digestibility, milk yield and milk composition of zebu cows. Trop Anim Health Prod. 2006;38:563-570.
22. Portnoy M, Barbano D. Lactose : Use , measurement , and expression of results. J Dairy Sci. 2021;104:2020-18706. http://dx.doi.org/10.3168/jds.2020-18706.
23. Silanikove N, Rauch-Cohen A, Shapiro F, Blum S, Arieli A, Leitner G. Lipopolysaccharide challenge of the mammary gland in bovine induced a transient glandular shift to anaerobic metabolism. J Dairy Sci. 2011;94:4468-4475. http://dx.doi.org/10.3168/jds.2010-4092.
24. Kvidera SK, Horst EA, Abuajamieh M, Mayorga EJ, Fernandez MVS, Baumgard LH. Glucose requirements of an activated immune system in lactating Holstein cows. J Dairy Sci. 2017;100:2360-2374. http://dx.doi.org/10.3168/jds.2016-12001.
25. Gross JJ, Grossen-Rösti L, Wall SK, Wellnitz O, Bruckmaier RM. Metabolic status is associated with the recovery of milk somatic cell count and milk secretion after lipopolysaccharide-induced mastitis in dairy cows. J Dairy Sci. 2020;103,5604-5615.
26. Albenzio M, Figliola L, Caroprese M, Marino R, Sevi A, Santillo A. Somatic cell count in sheep milk. Small Rumin Res.2019;176:24-30. https://doi.org/10.1016/j.smallrumres.2019.05.013.
27. Wall SK, Wellnitz O, Bruckmaier RM, Schwarz D. Differential somatic cell count in milk before, during, and after lipopolysaccharide- and lipoteichoic-acid-induced mastitis in dairy cows. J Dairy Sci. 2018;101:5362-5373. http://linkinghub.elsevier.com/retrieve/pii/S0022030218302510.
28. Bradley A, Green M. Use and interpretation of somatic cell count data in dairy cows. In Pract. 2005;27:310-315. http://inpractice.bmj.com/.
29. IDI. (International Dairy Federation). 2013. Guidelines for the use and interpretation of bovine milk somatic cell count. Bull. IDF. 466/2013. 2013;
30. INEN, Instituto Ecuatoriano Normalización. Raw milk. Requeriments.. 2012;1:2-7.https://www.gob.ec/sites/default/files/regulations/2018-10/Documento_BL NTE INEN 9 Leche cruda Requisitos.pdf.
31. Juozaitiene V, Juozaitis A, Micikeviciene R. Relationship between somatic cell count and milk production or morphological traits of udder in Black-and-White cows. Turkish J Vet Anim Sci. 2006;30:47-51.
32. Rakib MRH, Zhou M, Xu S, Liu Y, Asfandyar Khan M, Han B, et al. Effect of heat stress on udder health of dairy cows. J Dairy Res. 2020;87:315-321.
33. Collier RJ, Dahl GE, Vanbaale MJ. Major advances associated with environmental effects on dairy cattle. J Dairy Sci.2006;89:1244-1253. http://dx.doi.org/10.3168/jds.S0022-0302(06)72193-2.
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Guamán-Rivera S A, Guerrero-Pincay A E, Ortiz-Naveda N R, González-Marcillo R L and Herrera-Feijoo R J. Somatic Cell Count Evaluation in Early Lactation between Primiparous and Multiparous Bos indicus Cows. Revis Bionatura 2024; 9 (1) 16. http://dx.doi.org/10.21931/RB/2024.09.01.16
Additional information Correspondence should be addressed to santiagoa.guaman@espoch.edu.ec
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. First 13909355 Ecuador. Scopus coverage years: from 2016 to the present
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



















