Vol 8 No 4 2023 – 59
Antibacterial and Antifungal Activities of Apis mellifera L. Honey, Propolis, Royal Jelly in Iraqi Kurdistan Region
Banaz Abdulla1, Rukhosh Rashed2, Rebwar Hamasalih3, Tishk Shekh Faraj 4,*, Nashmil Rashid5, Hozan Hamamurad6
1 University of Salahaddin/ College of Education/ Department of Biology/ Erbil/ Iraq; banaz.abdulla@su.edu.krd.
2 University of Sulaimani/ College of Agricultural Engineering Science/ Department of Horticulture/ Sulaymaniyah/Iraq; rukhosh.rashid@univsul.edu.iq.
3 University of Salahaddin/ College of Education/ Department of Biology/ Erbil/ Iraq; rebwar.hamasalih@su.edu.krd.
4 University of Sulaimani/ College of Agricultural Engineering Science/ Department of Horticulture/Sulaymaniyah/ Iraq; tishk.shekhfaraj@univsul.edu.iq.
5 University of Sulaimani/ College of Agricultural Engineering Science/Department of Animal Science/ Sulaymaniyah/Iraq; nashmil.010016@univsul.edu.iq.
6 University of Salahaddin/ College of Agriculture Engineering Science/ Department of Plant Protection/Erbil/ Iraq; hozan.hamamurad@su.edu.krd.
* Correspondence: tishk.shekhfaraj@univsul.edu.iq.
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.65
ABSTRACT
This study was conducted at a laboratory in the Biology, College of Education, University of Salahaddin, from February to May 2022 to investigate propolis, honey and royal jelly’s chemical composition, antioxidant and antibacterial activities. The honeybee production extract showed that Gram (+) bacteria were more resistant to the antibacterial compounds of honey and propolis than Gram (-) bacteria and fungi. E. coli was a more sensitive isolate than all the other bacteria examined against the honey types tested. At the same time, it revealed more resistance against all types of propolis. Royal jelly with honey displayed more antimicrobial activity than other bee products and exhibited superior activity; the minimum inhibitory concentration of honey and propolis samples ranged from 32 to 512µg/mL. The MIC value of the most effective honey (Honey 1, Honey 2 and Royal jelly) was 32µg/mL. The lowest concentration of Qaladze propolis was (32µg/mL) for E. coli ATCC 25922, followed by 128µg/mL in some other propolis types.
Keywords: Apis mellifera; antimicrobial activities; honey; propolis; royal jelly.
INTRODUCTION
The honeybee (Apis mellifera L.) is the most common floral visitor. It is a critical insect that pollutes broad agricultural and wild plant species 1 sectors. In addition, bees were used as a source of unique, natural, multifunctional products such as honey, propolis and RJ 2. Bee products have been known for their nutritional and medicinal values from ancient up to the present time.
The natural sweetener (Honey) has a unique chemical structure, and its characteristics are affected by the plant’s source and geographical origin, as well as climatic, processing, and storage circumstances. Honey mainly comprises carbohydrates and water, affecting its shelf life and features like color, flavor, density, viscosity, hygroscopicity, and crystallization. Other components in honey include nitrogen compounds, minerals, organic acids, vitamins, volatile chemicals, and various bioactive molecules that alter sensory and physical properties and biological potential 3. An essential characteristic of honey is its multifactorial antibacterial action. 4. This property of honey has a fundamental relationship with the botanical origins of honey 5,6,7,8,9. Honey H2O2 is a key antibacterial component in honey. Under aerobic circumstances in diluted honey, H2O2 is created via glucose oxidase (GOX)-mediated glucose oxidation to gluconic acid 10.
Propolis is an adhesive product created by honeybees to guard and build their hives11. This product’s chemical compositions and biological properties are affected by several factors like sources of plants, geographical location, and collecting seasons 12,13,14. This sticky material has been used traditionally in many different human daily aspects such as home remedies, toothpaste, mouthwashes, creams, drops, dietary supplements, anti-putrefactive, antipyretic agents, antiseptic, wound healing agents, tuberculosis treatment, cold syndrome, treatment of burns, acne, herpes simplex and genitals, neurodermatitis, antifungal activities in ocular and vaginal infections 15. Kapare stated that raw propolis contains different components 16. Bankova divided bee glue into six types: poplar propolis, Brazilian green propolis, birch propolis, red propolis, pacific propolis and Canarian propolis 12. According to phytochemical screening, propolis contains anthraquinones, flavonoids, glycosides, alkaloids, fatty acids, saponins, triterpenes, tannins and volatile oils. In addition, pharmacologically, propolis can act as an antibacterial, antitumor, anticancer, antioxidant and anti-inflammatory 15. The constituents of 100gm of propolis according to comprise resins (50%), wax (30%), essential oils (10%), pollen (5%) and various organic compounds (5%) 16.
RJ is a creamy product secreted by the cephalic gland of worker bees to feed individual larvae in the hive in various proportions that performs a substantial role in caste differentiation 17,18. For the first three days, it is used to feed all individual larvae, and queen larvae are exceptionally longer and continue feeding, lasting five days 19-22. RJ comprises principally of water (60–70%), proteins (18%), carbohydrates (11%–23%), lipids (4%–8%), and mineral salts (1.5%) 23. This product is widely used in many areas, such as commercial medical products, healthy foods and cosmetics in various countries. It has been shown to have antibacterial, anti-inflammatory, vasodilative, hypotensive effects, disinfecting activity, antioxidant activity, anti-hypercholesterolemic activity, and anticancer characteristics 24. Pavel stated that RJ contains a particular protein known as a royalizing protein that acts as a solid antibacterial in vitro against Gram-positive bacteria17 due to the lack of study on the critical natural products Honey, Propolis, and RJ in Iraqi Kurdistan bees. The current study attempts to visualize the antimicrobial functions of these products.
MATERIALS AND METHODS
Propolis samples
Propolis samples were collected from A. mellifera hives at five different regions (Chami Rezan, Penjwen, Qaladze, Sharbazher, and Qandil) in the Sulaimani governorate, Iraqi Kurdistan region.
Extraction of the propolis samples
Raw propolis samples were dried, cleaned from impurities, ground to fine powders, weighed and mixed with 70% ethyl alcohol (1:5 w/v) in a sealed container at 37°C away from light with shaking twice a day for 20 days for extraction 25. Then, the supernatant liquid was filtered with Whatman No. 1 filter paper; the alcohol evaporated with a Rota vapor under a vacuum and was freeze-dried by lyophilizer. The samples were kept in a clean, airtight brown bottle in a refrigerator at -20 °C until use.
Honey sampling
Three samples of A. mellifera honey (fresh honey taken directly from the hives, honey purchased from the local markets, and honey with RJ) were collected from the study areas.
Honey sample preparation
Samples were prepared from crude honey and purified from bee wax, brood, and dead bees using sterile gauze. To study the susceptibility of microorganisms to different samples of honey, 75% (v/v) honey solution from the strained table honey was used.
Microorganisms test
Antimicrobial activities of the propolis and honey were carried out using the agar well diffusion technique. Three bacteria species (Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii) with their standard strains (S. aureus ATCC 25923, E. coli ATCC 25922, A. baumannii ATCC 19608) in addition to a fungus Candida albicans with its standard strain (C. albicans ATCC10231) were used in the current study. The strains were purchased from the Media Center in Erbil.
Assigning of antimicrobial activities of propolis and honey
The agar well diffusion test was achieved following the methodology of 26. A sterile cotton swab inoculated saline solution was used to culture bacteria and fungi in Potato dextrose agar plates and Mueller Hinton. The extra fluid was absorbed by plates left on the bench. A six-millimeter sterilized cork borer made a 4 mm deep well in the sealed agar medium. One hundred and fifty mL of honey and propolis (H1: Honey 1, H2: Honey 2, RJH: Royal jelly with honey, PCR: Propolis Chami Rezan, PP: Propolis Penjwen, PQ: Propolis Qaladze, PSH: Propolis Sharbazher, PQD: Propolis Qandil) in addition to the control were taken with a micropipette at various levels (12.5, 25, 50, 75, and 100%) which applied to the wells within the plates. Deionized water (negative control) and positive control (Ciprofloxacin (5 µg/mL) and fluconazole (25 µg/mL)) were put in the wells equally. The plates were incubated at 37°C for 24 hrs. One isolate for each species (A. baumannii, A. baumannii ATCC 19608, E. coli, E. coli ATCC 25922, S. aureus, S. aureus ATCC 25923, C. albicans, and C. albicans ATCC 10231) was assessed for its antimicrobial ability after 24 hours to evaluate the bee product effects on microbial growth. The inhibition zone diameters of the samples within the wells were measured with a caliper.
Minimum Inhibitory Concentration (MIC)
The identification of Minimum Inhibitory Concentration (MIC) was calculated using the broth microdilution method 26’27. The Mueller Hinton broth that showed a high inhibition zone against the test microorganisms was diluted by 3 mL or 3 µg/mL to create the stock honey solution (75%), which was used to evaluate the least effective antibacterial activity concentration. Based on the stock solution, a two-fold serial dilution was created. The turbidity of an 18–24 hrs. bacterial culture was compared with the 0.5 McFarland standard for standardizing and using. The 96 polystyrene wells microtiter plate detected the MIC against tested microbial strains. Then, 100 mL of different concentrations of propolis and honey (8, 16, 32, 64,128, 256, and 512) µg/mL from the stock honey solution were pipetted (1024 µg/mL) in a series of microtiter plate wells. 40 mL of the standardized inoculum suspensions were pipetted into each test well.
In contrast, the positive control well also included 40 mL of microorganisms for comparison purposes and the broth in the negative control well. After vortexing, the microtiter plate well was incubated for 24 hours at 37°C. Clear wells were utilized as the wells with the lowest propolis and honey concentrations that prevented bacterial development compared to the control wells.
DPPH radical scavenging activity
The free radical capturing activity of created compounds was measured using the method of 28,29. The ability of the compounds to scavenge electrons from DPPH (1,1-diphenyl-2-pierylhydrazyl) indicates their activities. Solutions of different concentrations (400, 600, 800 and 1000) µg/mL of the propolis, honey, and a standard solution of 0.004% of DPPH were stored in the dark. With different concentrations of the compounds, 2 mL of DPPH was mixed and left at room temperature in the dark for 60 min to complete the reaction. The mixture showed absorbance at 517 nm using a UV-visible spectrometer. The standard agent used was Ascorbic acid. The equation below was used to determine the percentage of inhibition.
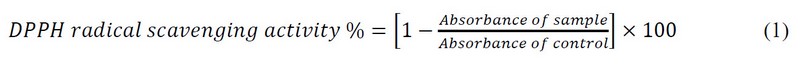
Statistical analysis
Antimicrobial activities were measured according to (IBM SPSS) statistics for Windows, version 26.0. at (p-value<0.05). Three replicate samples were used on two different occasions. Results were expressed as means ± standard error (M±SE). Mean values within a row with different letters (a–e) significantly differ for p-value < 0.05.
RESULTS
The antimicrobial activities of honey products were determined against Gram-positive and Gram-negative bacteria and fungi. Most samples showed measurable antibacterial activity, as shown in (Table 1).
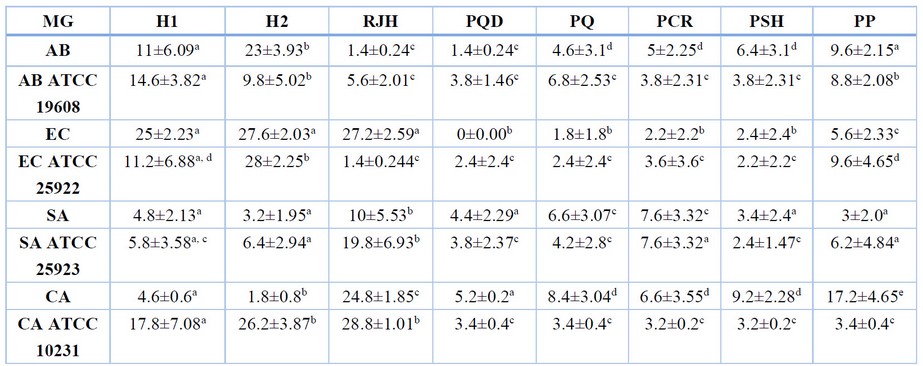
Table 1. In vitro antimicrobial activity of different honey and propolis against clinical microbial isolates. MG-Microorganisms; H-Honey; RJH-Royal Jelly Honey; PQD-Propolis Qandil; PQ-Propolis Qaladze; PCR-Propolis CHamiRezan; PSH-Propolis SHarbazher; PP-Propolis Penjwn; AB – Acinetobacter baumannii; EC – Escherichia coli; SA – Staphylococcus aureus; CA – Candida albicans.
Gram (+) bacteria were more resistant to the antibacterial compounds of honey and propolis than Gram (-) and yeasts. S. aureus among the Gram-positive and A. baumannii ATCC 19608 among Gram-negative bacteria were more resistant to the bee products tested. Furthermore, E. coli was more sensitive against the honey types than the others while revealing more resistance against all types of propolis.
Table and Figure 1 illustrate the statistical analysis of the bee product types’ antimicrobial activities (inhibition zone diameter (IZD) mm). RJH displayed more significant antimicrobial activity than other bee products and exhibited a superior activity (IZD range, 1.4–28.8%) compared to Honey 2 and Honey 1, which showed IZD ranges of 1.8-28% and 4.6-17.8%, respectively. A particular difference was recorded against C. albicans ATCC 10231 (IZD of RJH, 28.8%, vs. IZD of the other Bee products). However, the Qandil Propolis type was inactive against E. coli (IZD 0.00%) than the other species tested (IZD range, 1.4-5.2%).
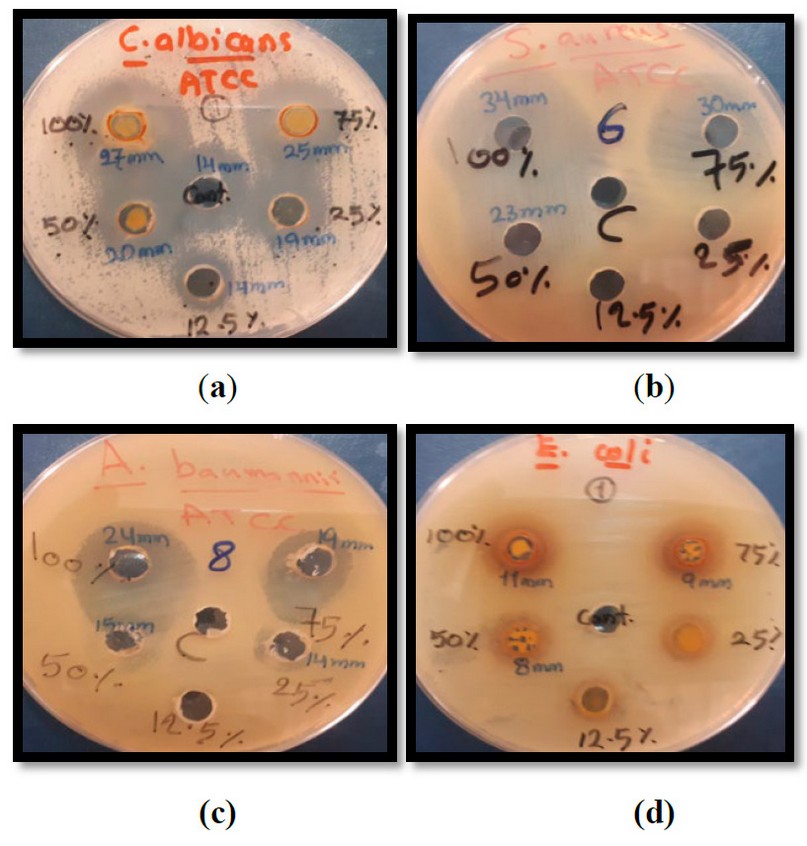
Figure 1. The diameter of inhibition zones (mm) of some pathogenic and standard microorganisms in different concentrations of different bee products. a – C. albicans; b – S. Aureus; c – A. baumannii; d – E coli.
When the growth of microbial isolates was established in the presence of bee product concentrations of 12.5, 25, 50, 75, and 100%, a significant reduction in growth was consistently observed at 100% concentrations of honey 2 against each of both strains of A. baumannii and E. coli (Figure 2). At the same time, Less effect was observed on growth against S. aureus and C. albicans compared to the control group. Remarkably, RJH was the most effective in preventing growth and was already active at 12.5-100% against S. aureus and C. albicans (p-value < 0.05) compared with the control. The RJH revealed less growth inhibition towards other microbial growth than the control.
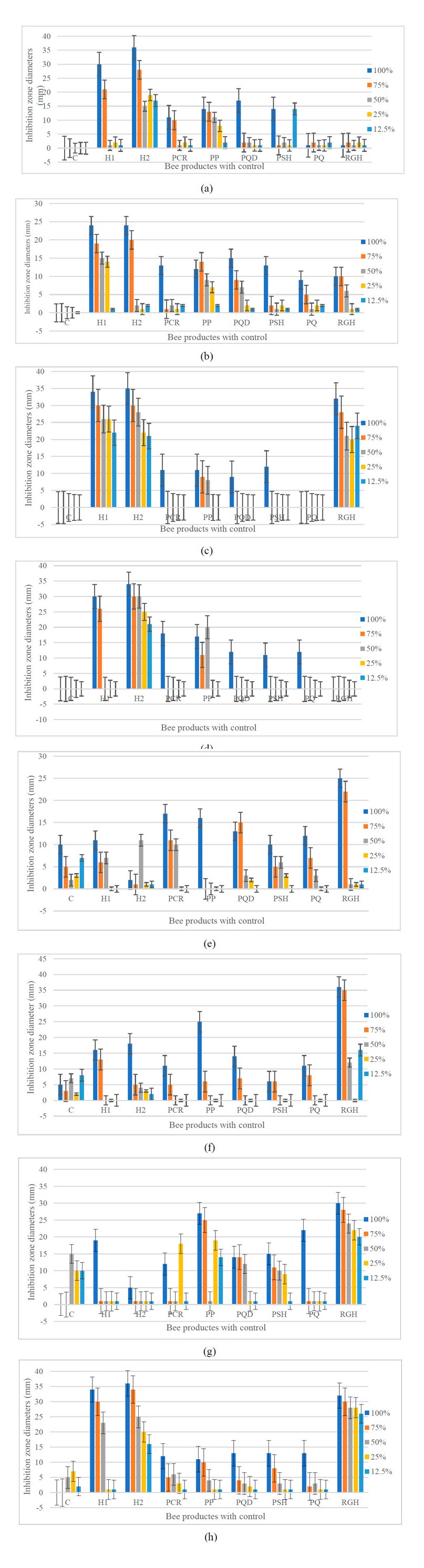
Figure 2. Effect of different types and concentrations of honey and propolis on microbial growth: Inhibition of microbial development against A. baumannii (a), A. baumannii ATCC 19068 (b), E. coli (c), E. coli ATCC 25922 (d), S. aureus (e), S. aureus ATCC 25923 (f), C. albicans (g), and C. albicans ATCC 10231 (h); Inhibition zone diameters of the concentration 100% (blue), 75% (orange), 50% (grey), 25% (yellow), and 12.5% (green) grown for 24 hours in Mueller-Hinton agar. Error bars represent means ± standard deviation (SD). Compared to the control, the dotted histograms represent non-significant values (p-value>0.05). C – control; H1 – Honey 1; H2 – Honey 2; PCR – Propolis Chami Rezan; PP – Propolis Penjwen; PQ – Propolis Qaladze; PSH – Propolis Sharbazher; PQD – Propolis Qandil; RGH – Royal Gel Honey.
Determination of MIC of bee products
The MIC of honey and propolis samples ranged from 32 to 512 µg/mL (Table 2). The MIC value of the most effective honey (Honey 1) was 32 µg/mL for A. baumannii ATCC 19608, E. coli ATCC 25922, S. aureus and S. aureus ATCC 25923. Also, the MIC of Honey 2 was 32 µg/mL for A. baumannii, A. the ATCC 19608, S. aureus, and S. aureus ATCC 25923, and the RJH recorded the same MIC value for E. coliATCC 25922, C. albicans and C. albicans ATCC 10231. The lowest concentration of propolis (32 µg/mL) was revealed in PQ for E. coli ATCC 25922, followed by 128 µg/mL in some other propolis types. The low concentrations of honey (diluted honey) used were effective at (32 µg/mL).
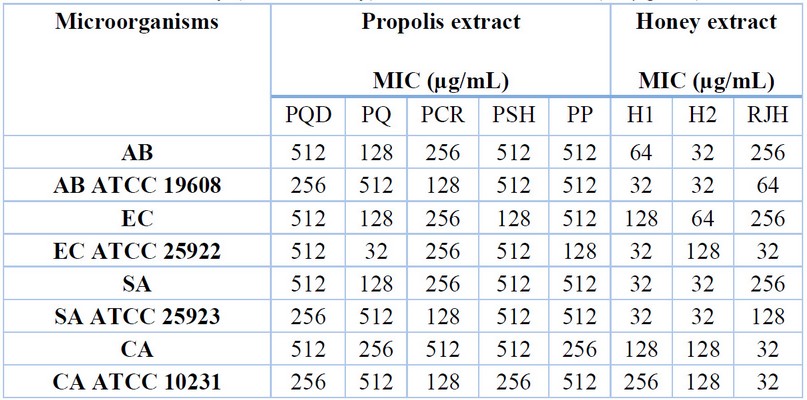
Table 2. Susceptibility of microbial strains to propolis and honey collected from diverse geographical locations in the Iraqi Kurdistan Region. AB – Acinetobacter baumannii; EC – Escherichia coli; SA ¬- Staphylococcus aureus; CA – Candida albicans; MIC – Minimum Inhibitory Concentration; PQD – Propolis Qandil; PQ – Propolis Qaladze; PCR – Propolis Chami Rezan; PSH – Propolis Sharbazher; PP – Propolis Penjwen; H1 – Honey 1; H2 – Honey 2; RJH – Royal Jelly Honey.
DPPH Radical Scavenging Activity
Figure 3 demonstrates the percentage of residual DPPH radical in the examined honeybee product samples. In this investigation, PP (IC50 = 140 µg/mL ) exhibited the most muscular DPPH radical scavenging activity with the lowest EC50, followed by RJH (IC50 = 190 µg/mL ), PQ (IC50 = 250 µg/mL), PCR (IC50= 375 µg/mL), and PQD (IC50 = 400 µg/mL). Honey 1 and 2 recorded the maximum value of IC50 (700 and 825 µg/mL, respectively). PSH shows the lowest DPPH radical scavenging activity with an IC50 value of 900 µg/mL. All findings were compared to Ascorbic acid standards’ IC50 value (150 µg/mL ). Therefore, PP’s antioxidant activity was more potent than the IC50 value of ascorbic acid.
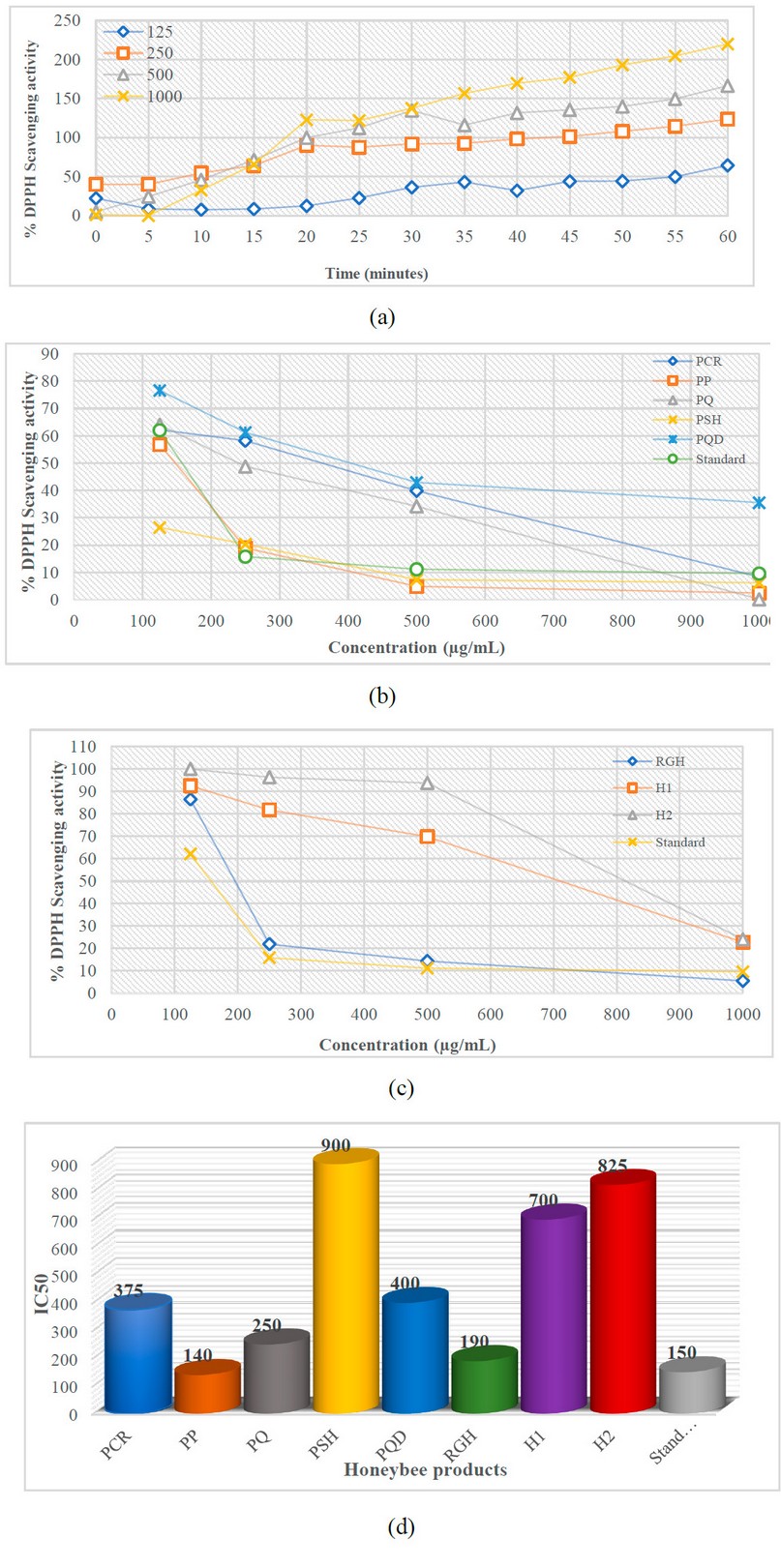
Figure 3. DPPH Radical Scavenging Activity; a. Effect of time on percentage DPPH inhibition at different concentrations, b and c. % of all compound’s inhibition at different concentrations, d. EC50 value of the honeybee products with the standard. H1 – Honey 1; H2 – Honey 2; PCR – Propolis Chami Rezan; PP – Propolis Penjwen; PQ – Propolis Qaladze; PSH – Propolis Sharbazher; PQD – Propolis Qandil; RGH – Royal Jelly Honey.
DISCUSSION
Propolis, honey and royal jelly bee products act as antifungal and antibacterial agents due to their contents of various biochemicals. The antimicrobial effect of honey could be explained by two mechanisms: hydrogen peroxide (H2O2) dependent and independent pathways. Concerning the first one, H2O2 is produced by glucose oxidase in honey, which metabolizes carbohydrates and causes the antimicrobial activity of honey. On the other hand, in the peroxide-independent pathway, the physical-chemical characteristics of honey, like high viscosity and sugar content, are considered the two factors giving honey the capability of antibacterial effect 30. Education of moisture in the environment causes bacterial dehydration by osmotic pressure. Studies show that in infant patients suffering from gastroenteritis, their recovery time is reduced significantly when given honey instead of glucose solution46. The reason may be due to the high sugar content in honey that improves electrolytes and water reabsorption in the intestine. In addition, the low PH level ceases microbial growth 31,32. Also, the results agree with 33, who indicated the antibacterial potency of some popular honey types such as Tualang and Manuka. Tualang, at low concentration, for instance, could be used to control the growth of some bacteria types such as S. Typhi, Shigella flexneri, and E. coli.
Pasupuleti found that propolis influences microorganism biological activities like permeability of the cellular membrane, producing ATP, breakdown of the membrane, and lowering the mobility of bacteria 34. 36 European propolis exhibited antifungal properties against some Candida strains, which agrees with our results; however, German propolis showed the weakest consequence on yeast cells (MFC> 5 µg/mL) 35. 37Peptides in RJ work as an antimicrobial agent, disrupting the structure of cell membranes. Fontana et al. 37demonstrated that RJ contains royalisin (antibacterial protein), which prevents it from contamination with Gram-positive bacteria.
Diverse diameters of inhibition zone (IZD) were recorded for the products. Differences in the activities of honey products may be due to their compositions and environmental factors such as geographical location, harvesting time, storage conditions, bee colony health, and age 38,39,40,41.
The honey product’s antimicrobial effectiveness against microbial growth after 24 hours was assessed here, and the RJH was recorded as superior to other products. The RJH comprises honey and royal jelly; this mixture strengthens RJH and acts as the strongest antipathogenic compared to the other products. This could be because honey and royal jelly contain various bioactive compounds that are more powerful against pathogens even at low concentrations 42,39.
Minimum inhibition concentration of the products exhibited significant results in some products and not in others. The lowest value of MIC was (32 µg/mL). This can be referred to as the activations of glucose oxidase enzymes and floral-origin catalase, which hydrolyze honey’s glucose to produce H2O2. This creates high oxidative stress, which is helpful in the determination of bacterial growth 10,43.
DPPH is a stable organic free radical that loses its absorption band at 517 nm when it accepts an electron or another free radical type. This experiment is widely employed to determine the antioxidant potential of natural products, especially honeybee components. The efficacy of honeybee products in reducing DPPH was assessed, and IC50 values were described as the quantity of honeybee product methanol extract required for a 50% reduction in DPPH. In addition, it can be stated as residual DPPH, which refers to the amount of unreduced DPPH radical 44. Our study revealed incredible results, especially for the product PP compared to the control. This outcome disagrees with Kurek et al.’s finding 45, which indicated that the EC50 of the control ascorbic acid was more effective than propolis. In contrast, the variations among different sources of propolis showed various DPPH radical scavenging activities. For example, the lowest EC50 was found in Turkish propolis (EC50 = 0.325 µg/mL), compared to Romanian propolis 1, 2, 3, and 4, which recorded (EC50 = 0.355 µg/mL, EC50 = 0.365 µg/mL, EC50 = 0.440 µg/mL, and EC50 = 0.460 µg/mL ) respectively.
CONCLUSIONS
In conclusion, Gram-positive bacteria are much more resistant to the antibacterial compounds of honey and propolis than Gram-negative bacteria and fungi. E. coli are more sensitive isolates than all other bacteria examined against the honey types tested while revealing more resistance against all types of propolis. RJ with honey displayed greater antimicrobial activities than the other types of bee products. The minimum inhibitory concentration of honey and propolis samples ranges from 32 to 512 µg/mL, while that of the most effective honey (Honey 1, Honey 2 and RJ) is 32 µg/mL. The lowest concentration of propolis types (32 µg/mL) is revealed in Qaladze propolis for E. coli ATCC 25922, followed by 128 µg/mL for other propolis types.
Author contribution:Conceptualization, Banaz Abdulla and Rebwar Hamasalih; Methodology, Banaz Abdulla and Rebwar Hamasalih; software, Rebwar Hamasalih; validation, Banaz Abdulla and Rebwar Hamasalih; Formal analysis; Rebwar Hamasalih; investigation, Rukhosh Rashid and Tishk Shekh Faraj; resources, Rukhosh Rashid and Tishk Shekh Faraj; data curation, Rebwar Hamasalih and Hozan Hamamurad; writing, Tishk Shekh Faraj, Banaz Abdulla and Rebwar Hamasalih; writing—review and editing, Tishk Shekh Faraj; visualization, Rukhosh Rashid; supervision, Banaz Abdulla, Rukhosh Rashid and Rebwar Hamasalih; project administration, Banaz Abdulla, Rukhosh Rashid, Rebwar Hamasalih, Tishk Shekh Faraj, Nashmil Rashid, Hozan Hamamurad. Funding acquisition, Banaz Abdulla, Rukhosh Rashid and Tishk Shekh Faraj. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Acknowledgment: The authors are grateful to the University of Salahaddin/College of Education/Departments of Biology and University of Sulaimani / College of Agricultural Engineering Science/department of Horticulture for providing vital support regarding the Laboratory and field. We also thank people from all studied sites for providing samples.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc R Soc B Biol Sci 2018, 285, (1870). doi:10.1098/rspb.2017.2140
2. Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and abiotic factors associated with colonies mortalities of managed honey bee (Apis mellifera). Diversity 2019, 11, 1–16. doi:10.3390/d11120237
3. Machado De-Melo, A.A.; Almeida-Muradian, L.B. de.; Sancho, M.T.; Pascual-Maté, A. Composición y propiedades de la miel de Apis mellifera: una revisión. J Apic Res 2018, 57, 5–37. doi:10.1080/00218839.2017.1338444
4. Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C enhances the antibacterial activity of honey against planktonic and biofilm-embedded bacteria. Molecules 2020, 25, doi:10.3390/molecules25040992
5. Adams, C.J.; Boult, C.H.; Deadman, BJ; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Corrigendum to Isolation by HPLC and characterization of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey Carbohydrate. Res. 2008, 343,(4), 651-659 (DOI:10.1016/j.carres.2007.12.011). Carbohydr Res. 344(18). doi:10.1016/j.carres.2009.08.008
6. Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res 2008, 52, 483–489. doi:10.1002/mnfr.200700282
7. Holt, S.; Johnson, K.; Ryan, J.; Catchpole, O.; Zhang, S.; Mitchell, K.A. New Zealand Kanuka Honey has high levels of methylglyoxal and antimicrobial activity. J Altern Complement Med 2012, 18, 203–204. doi:10.1089/acm.2011.0685
8. Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci Rep 2018, 8(1). doi:10.1038/s41598-018-27449-3
9. Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24. doi:10.3390/molecules24081573
10. White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. BBA – Biochim Biophys Acta 1963, 73, 57–70. doi:10.1016/0006-3002(63)90359-7
11. Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv Pharmacol Sci2013, 2013. doi:10.1155/2013/308249
12. Bankova, V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005,100, 1–2. doi:10.1016/j.jep.2005.05.004
13. Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J Ethnopharmacol. 2011, 133, 253–260. doi:10.1016/j.jep.2010.10.032
14. Popova, M.; Vassya, S. B.; Stefan, B.; Iva, T.; Christo, N.; Gian, Luigi, M., Anna-Gloria, S. Original article Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007, 38, 306–311.
15. Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
16. Al-Rawi, K.F., Ali, H.H., Guma, M.A., Alaaraji, S.F.T., Awad, M.M.The Relationships of Interleukin-33, Ve-Cadherin and Other Physiological Parameters in Male Patients with Rheumatoid Arthritis (2022) Pertanika Journal of Science and Technology, 30 (1), pp. 123-140.
17. Pavel, C.I.; Mărghitaş, L.A.; Bobiş, O.; Dezmirean, D.S.; Şapcaliu, A.; Radoi, I.; Mădaş, M.N. Biological Activities of Royal Jelly – Review. Sci Pap Anim Sci Biotechnol 2011, 44, 108–118. http://www.spasb.ro/index.php/spasb/article/view/560
18. Moritz, R.; Southwick, E.E.M. Bees as superorganisms: an evolutionary reality: Springer Science & Business Media, 2012.
19. Najim, Y. S. .; Mohammed, T. T. .; Hussain, F. M. . The Impact Of Varying Azolla Dosages On Male Broilers Diets In Terms Of Economic Feasibility And Physiologic Performance. JLSAR 2022, 3, 42-45
20. Shorter, J.R, Geisz, M.; Özsoy, E.; Magwire, M.M.; Carbone, M.A.; Mackay, T.F.C. The effects of royal jelly on fitness traits and gene expression in Drosophila melanogaster. PLoS One 2015, 10,1–10. doi:10.1371/journal.pone.0134612
21. Yang, W.; Tian, Y.; Han, M.; Miao, X. Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. PeerJ 2017, 2017. doi:10.7717/peerj.3118
22. Kunugi, H.; Ali, A.M. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int J Mol Sci 2019, 20,1–26. doi:10.3390/ijms20194662
23. Tokunaga, K.; hiko, Yoshida, C.; Suzuki, K, michi.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from Royal Jelly in spontaneously hypertensive rats. Biol Pharm Bull 2004, 27, 189–192. doi:10.1248/bpb.27.189
24. Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J Funct Foods 2012, 4, 39–52. doi:10.1016/j.jff.2011.12.007
25. Gonsales, GZ.; Orsi, R.O.; Fernandes, A.; Rodrigues, P.; Funari, S.R.C. Antibacterial activity of propolis collected in different regions of Brazil. J Venom Anim Toxins Incl Trop Dis 2006 12, 276–284. doi:10.1590/S1678-91992006000200009
26. Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac J Trop Biomed 2012, 2(7):554–557. doi:10.1016/S2221-1691(12)60096-3
27. Boateng, J.; Diunase, K.N. Comparing the antibacterial and functional properties of cameroonian and manuka honeys for potential wound healing-have we come full cycle in dealing with antibiotic resistance? Molecules 2015, 20. doi:10.3390/molecules200916068
28. Hamasalih, R.M.; Abdulrahman, ZFA Antibiofilm potency of ginger (Zingiber officinale) and quercetin against staphylococcus aureus isolated from urinary tract catheterized patients. Appl Ecol Environ Res 2020, 18,219–236. doi:10.15666/aeer/1801_219236
29. Samad, M.K.; Hawaiz, F.E. Synthesis, characterization, antioxidant power and acute toxicity of some new azo-benzamide and azo-imidazolone derivatives with in vivo and in vitro antimicrobial evaluation. Bioorg Chem 2019, 85,431–444. doi:10.1016/j.bioorg.2019.01.014
30. Ayoob, M.M.; Hussein, A.J.; Samad, M.K.; Dege, N.; Hawaiz, F.E.; Mohamed, S.K.; Hussain, F.H.S. Synthesis, Antibacterial and Antioxidant Activity of Azo-Oxazolone and Their Ring Opening Azo-Benzamide Derivatives. Curr Org Synth 2020, 18, 493–505. doi:10.2174/1570179417666201218163435
31. Nader, R.A.; Mackieh, R.; Wehbe, R.; Obeid, D. E,l.; Sabatier, J.M.; Fajloun, Z. Beehive products as antibacterial agents: A review. Antibiotics 2021, 10, 1–25. doi:10.3390/antibiotics10060717
32. Kwakman P.H.S, Velde AA te, Boer L, Speijer D, Christina Vandenbroucke‐Grauls MJ, Zaat SAJ. How honey kills bacteria. FASEB J 2010, 24, 2576–2582. doi:10.1096/fj.09-150789
33. Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation study of honey regarding their physicochemical properties and sugars and cyclitols content. Molecules 2020, 25, p 34. doi:10.3390/molecules25010034
34. Ramón-Sierra, J.; Martínez-Guevara, J.L.; Pool-Yam, L.; Magaña-Ortiz, D.; Yam-Puc, A.; Ortiz-Vázquez, E. Effects of phenolic and protein extracts from Melipona beecheii honey on pathogenic strains of Escherichia coli and Staphylococcus aureus. Food Sci Biotechnol 2020, 29, 1013–1021. doi:10.1007/s10068-020-00744-4
35. Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid Med Cell Longev2017,2017. doi:10.1155/2017/1259510
36. AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5,2. doi:10.3390/medicines5010002
37. Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple action mechanism and in vivo antimicrobial efficacy of antimicrobial peptide Jelleine-I. J Pept Sci 2021, 27. doi:10.1002/psc.3294
38. T. Jebril, N., Boden, R., Braungardt, C. Remediation Technique For Cadmium Contaminated Groundwater: A Systematic Review. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 1-18. doi: 10.32649/ajas.2023.178800.
39. Feknous, N.; Boumendjel, M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J Food Sci 2022, 40(3):163–178. doi:10.17221/247/2021-CJFS
40. Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; P-C.M.S. No Title Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem 2009, 112:1022–1030. doi:https://doi.org/10.1016/j.foodchem.2008.06.036
41. Chang, X.; Wang, J.; Yang, S.; Chen, S.; SY Antioxidative, antibrowning and antibacterial activities of sixteen floral honeys. Food Funct [Internet] 2011, 2:541–546. doi:https://doi.org/10.1039/C1FO10072F
42. Feknous, N.; Ouchene, L.L.; Boumendjel, M.; Mekhancha, D.E.; Boudida, Y.; Chettoum, A.; Boumendjel, A.; Messarah, M. Local honey goat milk yoghurt production. Process and quality control. Food Sci Technol 2022, 42:1–10. doi:10.1590/fst.26621
43. Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial activity of bee-collected pollen and beebread: State of the art and future perspectives. Antibiotics 2020, 9(11):1–29. doi:10.3390/antibiotics9110811
44. Zainol, M.I.; Mohd, Yusoff, K.; Mohd, Yusof.; MY Antibacterial activity of selected Malaysian honey. BMC Complement Altern Med 2013, 13. doi:10.1186/1472-6882-13-129
45. Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11,1–17. doi:10.3390/plants11091203
46. Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J Med Food 2010, 13, 605–609. doi:10.1089/jmf.2009.0075
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Abdulla B.; Rashed R.; Hamasalih R., Faraj T., Rashid N., Hamamurad H. Antibacterial and Antifungal Activities of Apis mellifera L. Honey, Propolis, Royal Jelly in Iraqi Kurdistan Region. Revis Bionatura 2023;8 (4) 65. http://dx.doi.org/10.21931/RB/2023.08.04.65



















