Vol 8 No 1 2023 – 51

Studying the toxicity of polluted water with polyaromatic hydrocarbon compound (Anthracene) by using micronucleus assay in fish
Milad A.Hussein1 , Estabraq N2
, Estabraq N2 . Abdul Lateef3
. Abdul Lateef3 , Sarab R. Mustafa4
, Sarab R. Mustafa4 , Noor Nihad Baqer4*
, Noor Nihad Baqer4* , Suha A. Ali5
, Suha A. Ali5 , Maha M Taen 6
, Maha M Taen 6 , Nora Saheab7
, Nora Saheab7
 , Estabraq N2
, Estabraq N2 . Abdul Lateef3
. Abdul Lateef3 , Sarab R. Mustafa4
, Sarab R. Mustafa4 , Noor Nihad Baqer4*
, Noor Nihad Baqer4* , Suha A. Ali5
, Suha A. Ali5 , Maha M Taen 6
, Maha M Taen 6 , Nora Saheab7
, Nora Saheab7
1,2,3,4,5,6,7 Ministry of Science and Technology / Environment And Water Director / Baghdad –Iraq
* Correspondence: : noornihadbaqer@gmail.com, mil76adali@gmail.com
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.51
ABSTRACT
Polycyclic aromatic hydrocarbons mainly originate from the incomplete combustion of fossil fuels such as petroleum, natural gas and coal. Also, biomass burning has attracted much attention due to its mutagenic, allergenic and carcinogenic properties. Anthracene, a three-ringed polycyclic aromatic hydrocarbon, is widely known as a common hazardous ubiquitous environmental pollutant. Anthracene is used to make dyes, plastics and pesticides.
The present study aims to evaluate the risks of Anthracene to fish using a micronucleus (MN) assay; the test has been used successfully as a mutagenic assay. Ninety fishes were adapted and acclimated to the laboratory conditions for one week before starting the experiment, then were exposed to (7.5mg/L, 10mg/L, and 12.5mg/L) of Anthracene for 72 hours. Results demonstrated that the LD50 of Anthracene in fish was (10 mg/L). Based on the values of LC50, the fish were then exposed for 72 h to three concentrations of sub-lethal Anthracene (2.5 mg/L, 5 mg/L and 7.5 mg/L) and control (0.00 mg/L) after (72 hours, 10 days, 20 days). Peripheral blood samples smears were collected from each group, the sample was stained by Giemsa stain, and frequencies of MNs were counted. The study showed an increase in micronuclei with concentration and period. In conclusion, it can use of the micronucleus assay in erythrocytes of fish as a sensible index for the assessment and evaluation of aquatic environmental pollution
Keywords: PAH; Anthracene; Micro nucleus assay; Carp.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAH) are a group of hydrophobic organic compounds that are widespread pollutants. Crude oil drops out, industrial, refinery activities, and civil wastes are essential sources of (PAH) in aquatic ecosystems.PAH are potentially carcinogenic agent 1 and a significant interest because of widespread contaminants in coastal and freshwater zones 2. PAH contamination is significant near industrialized areas 3.
Anthracene (C6H4.C2H2.C6H4) is a rigid polycyclic aromatic hydrocarbon with a low molecular weight derived from coal tar consisting of three fused benzene rings. Anthracene, Para naphthalene, is a Green Oil that accompanies naphthalene in the final steps of coal tar percolation. Anthracene is used in the artificial production of the red dye alizarin. It is also used in insecticides, coating materials and preservatives of wood. Moreover, Anthracene is colorless but exhibits blue fluorescence under ultraviolet light. If Anthracene is released into water, it will be expected to absorb particulate and sediments strongly. It was not hydrolyzed but may be bio-concentrated in aquatic organisms, which causes loss or reduction of microsomal oxidase (this enzyme enables the rapid metabolism of polyaromatic hydrocarbons). It undergoes immediate photolysis close to the surface of natural waters and maybe follows significant biodegradation based on laboratory experiments. Besides, industrial and agricultural activities have raised pollution in the aquatic environment, which is contaminated by different chemicals toxic from the discharge of waste waters and agriculture; these are accountable for several effects on the organisms, including humans 4. PAHs are absorbed by the fish body surface and via the gills. It also enters through food intake or contaminated materials 5.
Fish are an excellent topic for studying the mutagenic and/or carcinogenic possibility of contaminants in water samples since they can metabolize, concentrate and store waterborne pollutants 6. Since fish often react to toxicants in a similar way to higher vertebrates, they can be used to screen for chemicals that are potentially teratogenic and carcinogenic in humans. The main application of fish used as a test model is to mark the distribution and effects of chemical contaminants in the watery environment 7. Micronucleus assay was viable to freshwater and marine fishes and showed that gill cells are more sensitive than hematopoietic cells to micronucleus-inducing agents. The micronucleus assay is an in vivo and in vitro short-time screening procedure that is openly used to discover genotoxic effects. It is one of the most straightforward, dependable, low expenses and fast screening systems 8. The MN test, one of the most common tests of environmental genotoxicity, has served as an indicator of cytogenetic hurt 9. Micronuclei are cytoplasmic chromatin masses with the appearance of small nuclei that emerge from chromosome fragments or intact total chromosomes lagging in the anaphase phase of cell division. Their existence in cells reflects structural and numerical chromosomal aberrations arising through mitosis 10. These compounds may create hepatic lesions and physiological and biochemical disorders in fish. Contaminated fish may then be used to bio-observe the existence and importance of these pollutants. The present study aims to evaluate the risks of Anthracene to fish by using a micronucleus (MN) assay,
MATERIALS AND METHODS
There are 90 fish of common carp (Cyprinus carpio) ranging between 7-9 cm in total length and 60-80 gm. in body weight, with no visible signs of disease or morbidity. Fish were kept in an aquarium with aerated tap water; they were fed on commercial feed and adapted to laboratory conditions for one week before the experiments were complete.
The experiment was conducted in a 20 L aquarium and gently aerated tap water. A set of 10 fish specimens were randomly exposed to anthracene concentrations (7.5, 10.0 and 12.5mg/L); after 72 h, the value of LC50 of Anthracene for C. carpio was specified as 10.0 mg/L.
Based on the LD50 values, the fish were then exposed for 72 h to three concentrations of sub-lethal Anthracene (2.5mg/L, 5mg/L and 7.5 mg/L) and control (0.00 mg/L). The mortalities did not occur in all groups following the period of exposure. After 72 h (Acute exposure), the water was exchanged, and the blood was collected from the caudal vein for a micronucleus test after 72 hrs, 10 and 20 days of the experiment. Statistical analysis was performed by using Excel word 2019
Micronucleus Test
The smear of peripheral blood pulled from the caudal vein by a syringe coated with heparin was prepared, and well-dried slides were stained with 10% Giemsa stain solution for a half hour following the method of 11. (1000 cells/slide) for micro-nuclei were scored.
RESULTS
The results of this study showed that the MN was increased, correspondingly with the increasing anthracene concentrations (2.5%, 5%, and 7.5%) than that control group in all the anthracene concentrations (Figure 1).
Micronucleus was calculated in the acute group (after 72 hrs), and the chronic group (after 10 and 20 days of the experimental period) showed an increase of micronuclei with the increase in concentration, and also when compared within the group according to the period of exposure as in Figure (2,3,4,5).
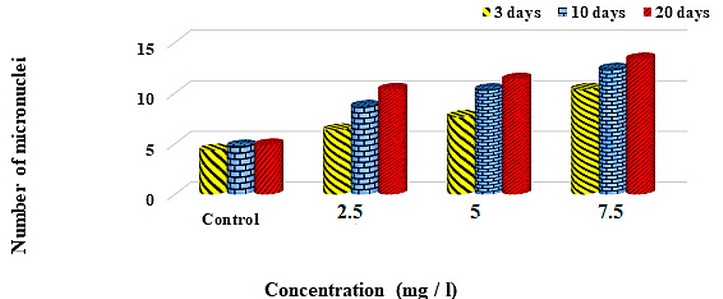
Figure 1. The number of micronuclei at different times and concentrations of Anthracene (mg/L).
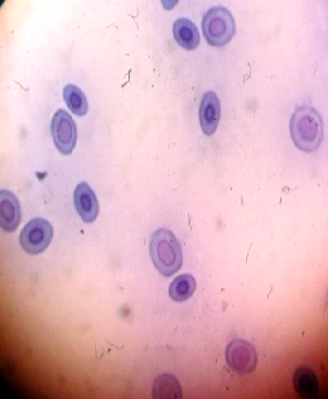
Figure 2. Normal red blood cells without micronuclei
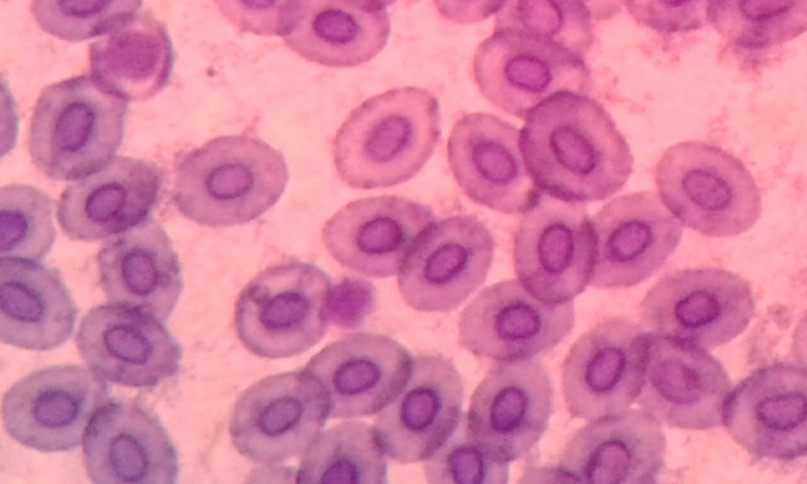
Figure 3. Micronuclei in Red Blood Cells of Fish
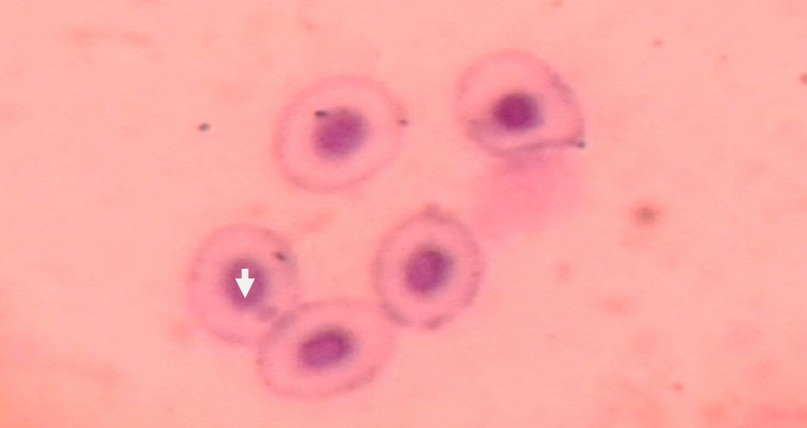
Figure 4. Micronuclei in Red Blood Cells of Fish
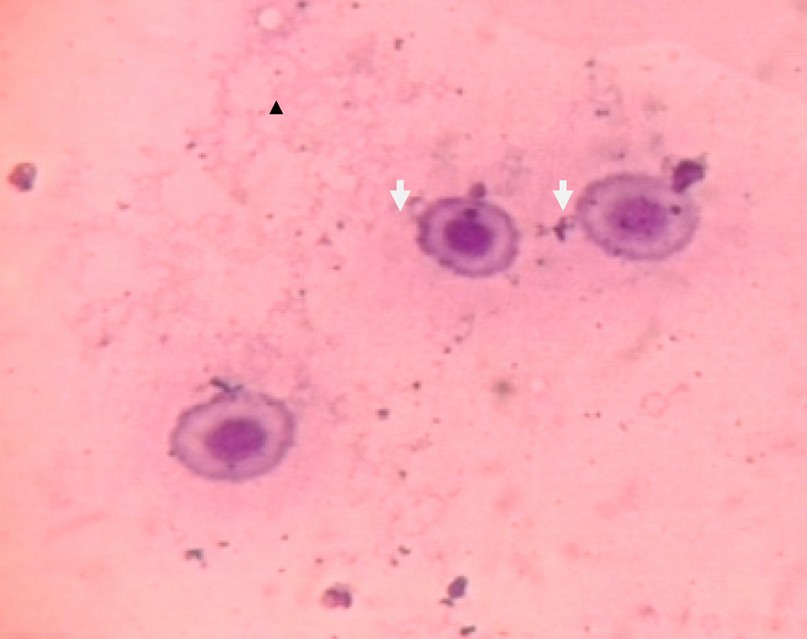
Figure 5 Micronuclei in Red Blood Cells of Fish
DISCUSSION
The results agreed with the development of Carlos et al. 12, who observed an increase in clastogenic accidents, and submission of the formation of MN when considering nuclear alterations and micronuclei together; group xenobiotic exposure and controls were significantly different.
Micronuclei occur due to exposure to different concentrations of benzene and Anthracene. The effect of these structures on the chromosome to form chromosome fragments or loss of centromere lead to delay of these fragments in the anaphase stage cell mitosis. After the telophase stage, these chromosome fragments are turned around to form Micronuclei 13,14.
Differing from other organisms belonging to the trophic chain, fishes are sensitive to relatively small concentrations of environmental Pollutants (with the possibility of mutagenic effects); thus, fishes are considered excellent bioindicators of environmental biomonitoring 15.
MNs assay, initially developed in mammalian species, has been widely used to evaluate the genotoxic effect of many chemical agents 16.
MNs assay can be considered a useful bioindicator of genotoxic and cytotoxic effects of contaminants in aquatic organisms 17,18,19,20.
The results showed the importance of MNs assay in mutagenic altered assessment in fishes, which can be used as sentinel organisms to indicate the potential for human exposure to genotoxic chemicals in drinking water. The concentrations of contaminants in fish reverse the situation of contamination of the environment, and thus, the observed levels of total PAHs in fish indicate high levels of PAHs contamination. However, the Consumption rate of 1 g/day appears to be protective from the carcinogenic effects of the PAHs levels. This is because the (Predicted Environmental Concentration) PEC values associated with a consumption rate of 1 g/day are found to be less than the screening value 21
The present study showed that high PAH levels affect the health of fish and, thus, consumption of these fish may cause a significant health risk.
CONCLUSIONS
The present study showed that high PAH levels affect the health of fish and, thus, consumption of these fish may cause a significant health risk. It can use the micronucleus assay in erythrocytes of fish as a sensible index for assessing and evaluating aquatic environmental pollution.
Author Contributions: All authors contributed to the design methodology, analysis results and writing manuscript
Funding: No funding
.
Acknowledgments: We thank the Ministry of Science and Technology / Environment and Water Director / Baghdad /Iraq.
Conflicts of Interest: There is no conflict
REFERENCES
1. Shailaja MS, D’Silva C. Evaluation of impact of PAH on a tropical fish, Oreochromis mossambicus using multiple biomarkers. Chemosphere. 2003 Dec 1;53(8):835-41.
2. Akaishi FM, Silva de Assis HC, Jakobi SC, Eiras-Stofella DR, St-Jean SD, Courtenay SC, Lima EF, Wagener AL, Scofield AL, Oliveira Ribeiro CA. Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Archives of environmental contamination and toxicology. 2004 Feb;46(2):244-53.
3. Aas A, Beyer J, Goksoyr A. Fixed wavelength fluorescence (FF) of bile as a monitoring tool for polyaromatic hydrocarbon exposure in fish: an evaluation of compound specificity, inner filter effect and signal interpretation. Biomarkers. 2000 Jan 1;5(1):9-23.
4. Isani G, Andreani G, Cocchioni F, Fedeli D, Carpene E, Falcioni G. Cadmium accumulation and biochemical responses in Sparus aurata following sub-lethal Cd exposure. Ecotoxicology and environmental safety. 2009 January 1;72(1):224-30.
5. Vasanth S, Ganesh A, Vijayakumar TS, Karthikeyeni S, Manimegalai M, Subramanian P. Assessment of Anthracene on hepatic and antioxidant enzyme activities in Labeo rohita (Hamilton, 1822). International Journal of Pharmacy & Life Sciences. 2012 May 1;3(5).
6. Al-Sabti K, Metcalfe CD. Fish micronuclei for assessing genotoxicity in water. Mutation Research/Genetic Toxicology. 1995 Jun 1;343(2-3):121-35..
7. Al-Sabti K. Handbook of genotoxic effects and fish chromosomes. Handbook of genotoxic effects and fish chromosomes.. 1991.
8. Hayashi M, Ueda T, Uyeno K, Wada K, Kinae N, Saotome K, Tanaka N, Takai A, Sasaki YF, Asano N, Sofuni T. Development of genotoxicity assay systems that use aquatic organisms. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1998 Mar 20;399(2):125-33.
9. Baršienė J, Dedonytė V, Rybakovas A, Andreikėnaitė L, Andersen OK. Investigation of micronuclei and other nuclear abnormalities in peripheral blood and kidney of marine fish treated with crude oil. Aquatic Toxicology. 2006 Jun 1;78:S99-104.
10. Bolognesi C, Hayashi M. Micronucleus assay in aquatic animals. Mutagenesis. 2011 Jan;26(1):205-13.
11. Schmid W. The micronucleus test. Mutat Res. 1975 Feb;31(1):9-15.
12. Da Rocha CA, Dos Santos RA, Bahia MD, Da Cunha LA, Ribeiro HF, Burbano RM. The micronucleus assay in fish species as an important tool for xenobiotic exposure risk assessment—a brief review and an example using neotropical fish exposed to methylmercury. Reviews in Fisheries Science. 2009 Oct 2;17(4):478-84.
13. Heddle JA, Salamone MF. Chromosomal aberrations and bone marrow toxicity. Environmental Health Perspectives. 1981 Jun;39:23-7.
14. Al-Aadhhami SM .Studying Chromosomal abnormality of human induced by petroleum by product. Master thesis, college of Science, Baghdad University.2000.
15. Minissi S, Ciccotti E, Rizzoni M. Micronucleus test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: a bioassay for the in situ detection of mutagens in freshwater. Mutation Research/Genetic Toxicology. 1996 Apr 6;367(4):245-51.
16. Dailianis S, Domouhtsidou GP, Raftopoulou E, Kaloyianni M, Dimitriadis VK. Evaluation of neutral red retention assay, micronucleus test, acetylcholinesterase activity and a signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Marine Environmental Research. 2003 Oct 1;56(4):443-70.
17. Ferraro MV, Fenocchio AS, Mantovani MS, Ribeiro CD, Cestari MM. Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genetics and Molecular Biology. 2004;27:103-7.
18. Cavas T, Garanko NN, Arkhipchuk VV. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food and Chemical Toxicology. 2005 Apr 1;43(4):569-74.
19. Matsumoto ST, Mantovani MS, Malaguttii MI, Dias AL, Fonseca IC, Marin-Morales MA. Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genetics and Molecular Biology. 2006;29:148-58.
20. Lanfranchi AL, Menone ML, Miglioranza KS, Janiot LJ, Aizpun JE, Moreno VJ. Striped weakfish (Cynoscion guatucupa): a biomonitor of organochlorine pesticides in estuarine and near-coastal zones. Marine Pollution Bulletin. 2006 Jan 1;52(1):74-80.
21. Nyarko E, Klubi BE. Polycyclic aromatic hydrocarbons (PAHs) levels in two commercially important fish species from the coastal waters of Ghana and their carcinogenic health risks. West African Journal of Applied Ecology. 2011;19(1).
Received: December 23, 2022 / Accepted: January 30, 2023 / Published:15 February 2023
Citation: Hussein M A, Abdul Lateef E N, Mustafa S ., Baqer N N, Ali S A., Taen M M, Saheab N.
Study the toxicity of polluted water with polyaromatic hydrocarbon compound (Anthracene) using micronucleus assay in fish. Revis Bionatura 2023;8 (1) 51. http://dx.doi.org/10.21931/RB/2023.08.01.51



















