Vol 8 No 1 2023 – 34

Inhibitory effect of Titanium dioxide (Tio2) nanoparticles and their synergistic activity with antibiotics in some types of bacteria
1 Department of Biology, College of Science, Mosul University, Mosul, Iraq
email : ashsbio102@ uomosul.edu.irq
2Department of Biology, College of Science, Mosul University, Mosul, Iraq
email: imanalkhayyat@uomosul.edu.iq
3Department of Biology, College of Science, Mosul University, Mosul, Iraq
email: kaysbio106@uomosul.edu.iq
*Correspondence: ashsbio102@ uomosul.edu.irq
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.34
ABSTRACT
Titanium dioxide nanoparticles (TiO2 NPs) were studied as antibacterial agents at different concentrations against clinical and environmental bacterial isolates without UV or photocatalytic activation. Five TiO2 NPs concentrations (20µg/ml,50µg/ml, 100µg/ml,500µg/ml and 1000µg/ml) were studied against 15 bacterial species:10 clinical isolates and 5 environmental isolates) compared with antibiotics Amikacin(AK)and Levloxacin(LEV).Only500µg/ml concentration of TiO2 NPs was active against 7 bacterial isolates (3 clinical and 4 environmental), and 1000µg/ml concentration of TiO2 NPs was effective against 9 isolates (6 clinical and 3 environmental ). These concentrations were mixed with the antibiotics Levloxacin LEV and Amikacin AK to investigate the possibility of synergistic activity against studied bacteria. Bacterial isolate’s response or sensitivity to the antibiotic and TiO2 NPs mixture was varied; AK plus 500µg/ml TiO2 NPs concentration showed increased inhibitory activity against 7 isolates (3 clinical, 4 environmental) and 1000µg/ml TiO2 NPs mixed with AK showed increased inhibition activity against one environmental bacterial isolates, where Ak mixed with 500 and Ak plus 1000 µg/ml showed the same effect as the antibiotic alone or less.LEV antibiotic shows no difference in the effect on all 9 bacteria (7 clinical and 2 environmental), while LEV mixed with 500 µg/ml have increased inhibition zones on 4 bacteria (2 clinical, 2 environmental) , and LEV mixed with 1000µg/ml have higher effect than the antibiotic alone on three isolates (2 clinical,1 environmental).
Keywords: antibiotic; titanium nanoparticles dioxide; antibacterial.
INTRODUCTION
The mechanical properties of titanium-based implants have improved significantly in recent years thanks to using TiO2 NPs in medical devices. Overcome the bio inertness of the raw metals, corrosion resistance and the rate at which metal ions are released (to prevent aseptic loosening of the implant)1.
In the early stages of the «race for the surface,» the implant may get infested with bacteria. Aseptic loosening of implants and biomaterial-centered infections (BCI) play a significant role in prosthetic implant failure and infection of the implant itself, making them a severe medical problem2
In addition to its photocatalytic properties, TiO2 NPs can be used for water separation, energy production, air and water purification and surface sterilizing, organic compound synthesis, and pollution reduction(TiO2 can be used as an adsorbent for environmental pollutants, where it deals with air pollutants such as dust and dust and adsorbs them on the surfaces on which they fall. As for water pollutants, they can be used as a precipitant for contaminants that contain water.)3 Nanoparticles (NPs) are also employed to make medications, detect infections, proteins, and tumors, and separate and purify biological components and cells4,5. Titanium dioxide (TiO2) inorganic nanoparticles have been used for decades due to their non-toxicity, ease of production, and low cost. Furthermore, it may have a universal bactericidal mechanism6. The morphological characteristics of titanium dioxide NPs significantly impact their applications7. Rutile, anatase, and brookite are all types of TiO2 NPs that can be found in nature. Of the three types of photocatalysts mentioned, anatase NPs are the most commonly used8,9.
Many people are interested in the antibacterial characteristics of TiO2 in the food industry. TiO2 has been declared nontoxic by the FDA in the United States. TiO2 has been Many people are interested in the antibacterial characteristics of TiO2 in the food industry right now. TiO2 has been declared nontoxic by the FDA in the United States10. In addition to its fungicidal activity, TiO2 NPs have been demonstrated to exhibit bactericidal effects on bacteria such as E. coli, Staphylococcus aureus, and Pseudomonas putida11,12. There has been some interest in food packaging with TiO2-coated or incorporated TiO213,14. For food packaging, antimicrobial agents combat germs and enhance traditional packaging functions, such as shelf life extension, quality preservation, and safety assurance15. Using an antibacterial agent helps to reduce the spread of hazardous germs and keep food fresh, as shown in figure (1)16,17
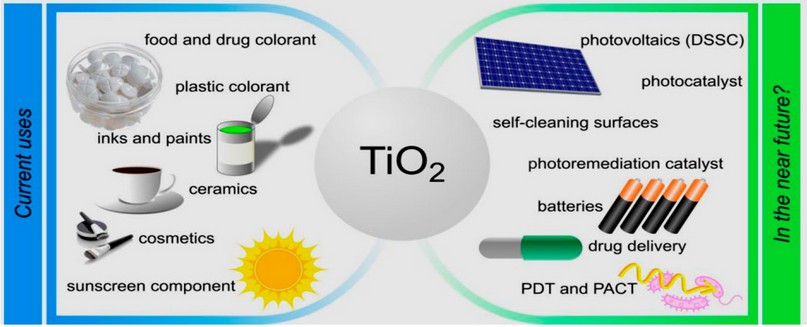
Figure 1. Applications of TiO2 NPs and the perspective shortly. DSSC( Dye-sensitized solar cell); PACT (antimicrobial photodynamic therapy); PDT (photodynamic therapy)18.
The anatase form of titanium dioxide nanoparticles (without UV activation or photocatalytic activation) was examined for its antibacterial activity against pathogenic and environmental Gram-positive and Gram-negative bacteria to assess its application as a new antibacterial approach or for ecological health.
MATERIALS AND METHODS
Nanoparticles
The Canadian company MK impex corp supplies commercial TiO2 nanoparticles with a size of 100nm. Were used in this study.
Bacterial Isolates
A total of 15 bacterial isolates were used, which included 7 clinical isolates obtained from wound and urine infections and 6 environmental isolates from water, as shown in table( 1 ).
All isolates were identified by standard microbiological procedures (Gram staining, colonial morphology, catalase test, cytochrome oxidase reaction, motility, and other biochemical tests), which were carried out depending on Bergey’s manual of systematic Bacteriology, also by Vitek 2 system19
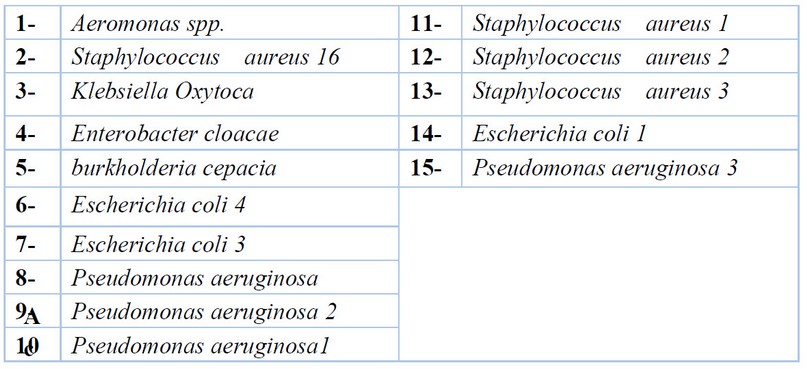
Table 1. Bacterial isolates were used in this study.
Use of TiO2 NPs as a bactericidal inhibitor of:
For testing the antibacterial activity, five concentration of TiO2 NPs were prepared by dissolving the NPs powder in deionized distilled water20; different dilution was prepared, including (1000 µg/ml,500 µg/ml,100µg/ml, 50 µg/ml,20 µg/ml )
This preparation was mixed using a vortex for 3 minutes to obtain homogeneous dilution. Whatman no. 1 filter paper discs were saturated with each concentration of TiO2 NPs and used in the sensitivity test.
Antibiotic Amikacin AK (10 mcg) and Lefloxacin LEF (5 mcg) supplied by Bioanalyzer (turkey) were used as positive control for each bacterium.
Bacterial inoculums were prepared by Direct Colony Suspension Method (1-3). Isolation colonies were selected and transferred to a tube of nutrient broth mixed well using a vortex; the bacterial no was fixed at 1.5×108 by comparing the turbidity with the 0.5 McFarland standard,21. Mueller-Hinton agar plates were inoculated with the suspension of each bacterial strain, then five TiO2 NPs discs, as well as the antibiotic discs, were distributed unit formally on the agar surface, then incubated at 37°C for (20-24) h. Inhibition zones were measured to monitor the effects of TiO2 NPs on bacterial growth22. Positive results were scored when a zone of inhibition was observed around the discs after incubation. The experiments were performed in triplicate to obtain means values for each bacterial isolates9 .
Synergistic effects between the active concentrations of TiO2 NPs and the antibiotics against bacteria:
The conc.1000 µg/ml and 500 µg/ml were chosen and mixed with the standard antibiotic discs by adding 250 µl from each concentration to 15 antibiotic discs after the experiment in 15 minutes.The antibiotic sensitivity disc was done according to CLSI. Each experiment was repeated three times.
RESULTS
The activity of five concentrations of TiO2 nanoparticles is shown in table (2), as well as control antibiotics AK and LEV against 15 bacterial isolates from clinical and environmental sources. The concentrations (20, 50, 100) µg/ml didn’t show any antibacterial against all bacteria, while the concentration of 500µg/ml of TiO2 nanoparticles shows inhibitory activity against 7 isolates (3 clinical and 4 environmental including Aeromonas spp., Klebsiella Oxytoca, Pseudomonas aeruginosa 1
Staphylococcus aureus 1, Staphylococcus aureus 3, Escherichia coli 1, Pseudomonas aeruginosa 3), the 1000µg/ml showed antibacterial activity against 9 isolates (6 clinical isolates including Aeromonas spp., Klebsiella oxytoca, Enterobacter cloacae, Burkholderia cepacia, Escherichia coli 4, Escherichia coli 3 and 3 environmental isolates including Stapylococcus aureus 1, Staphylococcus aureus 3, Escherichia coli 1 ).
The inhibitory effect of these two concentrations differs against the different bacterial isolates used, and it also concerns varying the diameters of the inhibitory zone.
The concentration of 500µg/ml of nanoparticles showed a higher inhibition zone than AK antibiotic on 4 isolates (2 clinical including Staphylococcus aureus 16, Escherichia coli 4 and 2 environmental isolation Staphylococcus aureus 1, Staphylococcus aureus 3). In contrast, the antibiotic LEV has the highest inhibitory effect against all bacterial isolates. Also, the concentration 1000 µg/ml showed a higher impact than AK on two bacteria (1 clinical Aeromonas spp. and 1 environmental Escherichia coli 3 isolates). Diagrams (1,2,3 and 4)illustrate the antibacterial activity of different TiO2 NPs concentrations against studied bacteria.
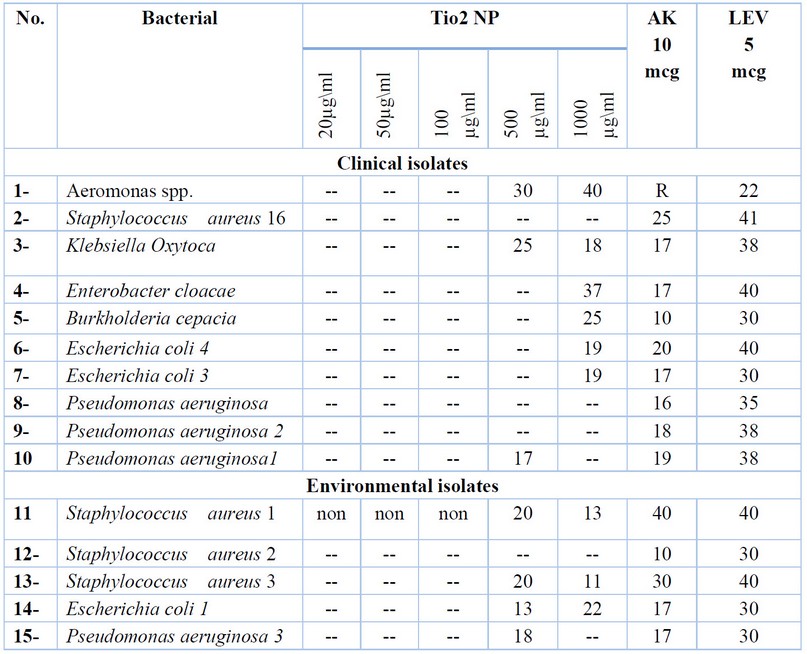
Table 2. the effectiveness of different concentrations of TiO2 NP compared with the antibiotics LEV, AK.
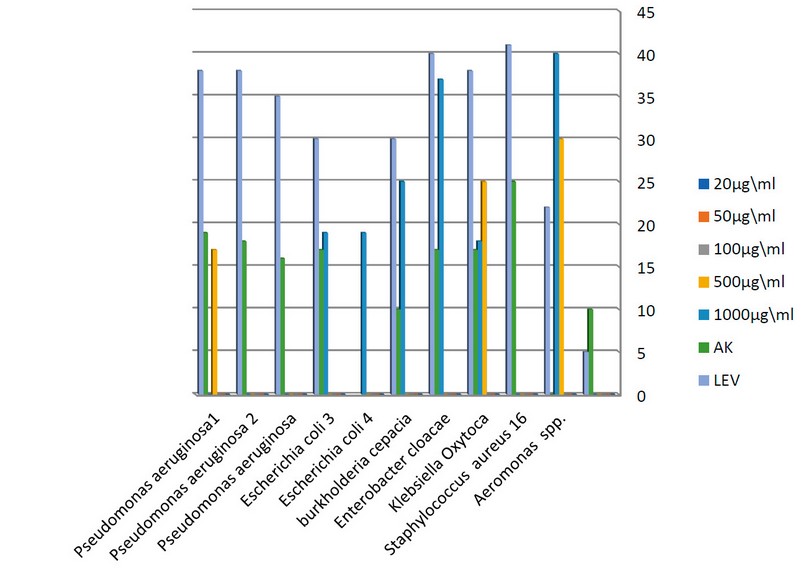
Figure 2. the effect of different concentrations of tio2 NPs on Clinical bacteria.
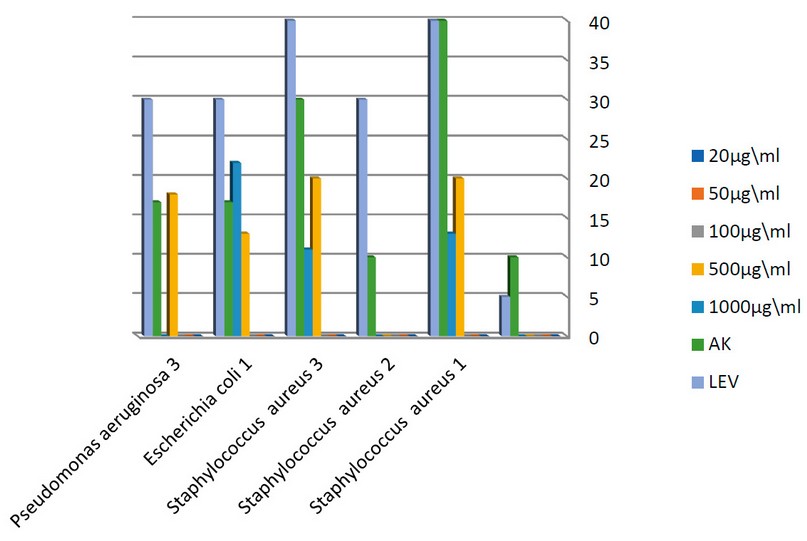
Figure 3. the effect of different concentrations of TiO2 NPs on environmental bacteria.
3Study the synergistic activity between antibiotics and TiO2 nanoparticles:
500 and 1000µg/ml TiO2 nanoparticles concentrations were mixed with each antibiotic AK and LEV and studied against bacteria.AK plus the concentration 500µg/ml TiO2 showed an increased inhibitory zone against 7 isolates (3 clinical Staphylococcus aureus 16, Enterobacter cloacae, Pseudomonas aeruginosa,4 environmental Staphylococcus aureus 1, Staphylococcus aureus 2, Staphylococcus aureus 3, Escherichia coli 1) on mixing the concentration 1000µg/ml TiO2 nanoparticles with AK antibiotic shows causes an increase in the inhibition zone against(Staphylococcus aureus 1, Staphylococcus aureus 2, Staphylococcus aureus 3, Escherichia coli 1, Pseudomonas aeruginosa 3, where is the other bacterial response to AK.AK mixed with 500 and AK with 1000 µg/ml causes no significant difference.
LEV antibiotic showed no difference in its effect on (7 clinical Aeromonas spp., Staphylococcus aureus 16, Klebsiella oxytoca, Enterobacter cloacae, Burkholderia cepacia, Escherichia coli 4, Escherichia coli 3 and 3 environmental Staphylococcus aureus 1, Staphylococcus aureus 2, Staphylococcus aureus 3),
while LEV mixed with 500 µg/ml causes increased inhibitory zones on ( Burkholderia cepacia, Escherichia coli 3, Staphylococcus aureus2, and Pseudomonas aeruginosa 3) , and LEV mixed with 1000µg/ml increased the effect antibiotic effect on (2 clinical Burkholderia cepacia, Pseudomonas aeruginosa and 1 environmental Pseudomonas aeruginosa3) and the diagrams (3,4) illustrate the synergistic effect on TiO2 NPs and the chosen concentration of antibiotics.
Fig: (1 and 2) showed the antagonistic effect of AK, LEV and the synergistic effect with concentrations of TiO2 NPs on the Staphylococcus aureus and Klebsiella oxytoca bacteria as showed in table (3).
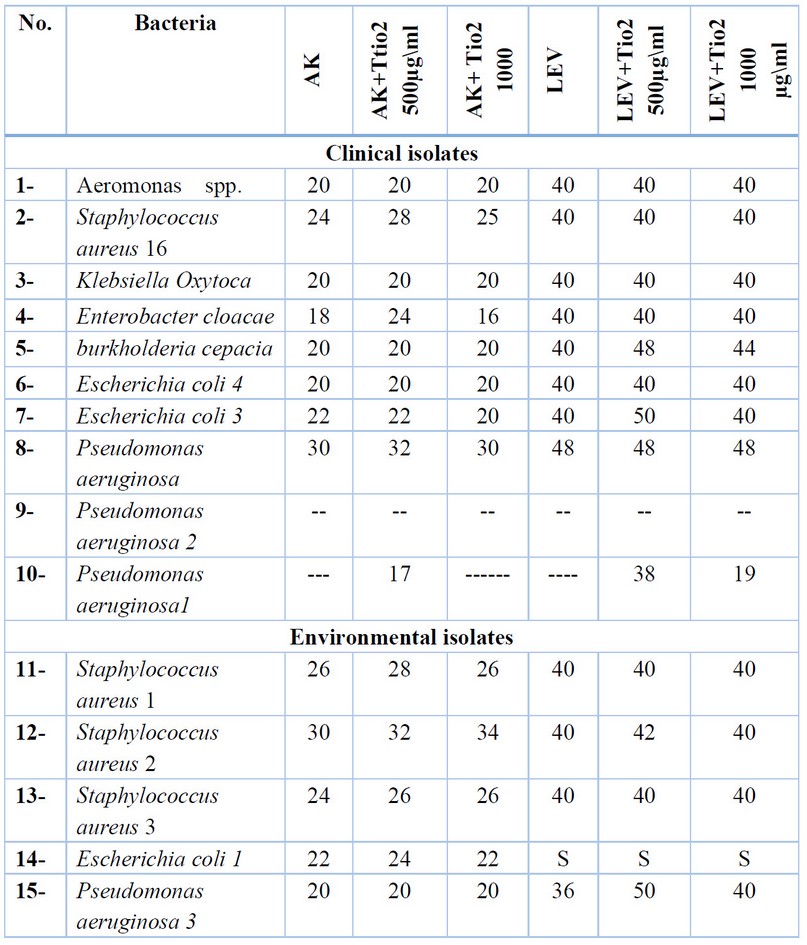
Table 3. Synergistic result between tio2 and the antibiotics (LEV, AK).
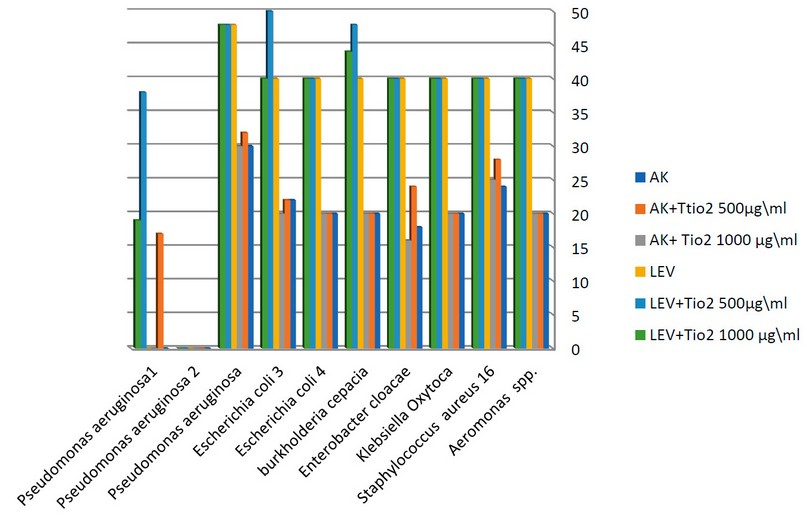
Figure 4. The synergistic effect of at TiO2 NPs a chosen concentration with of antibiotics against clinical bacterial isolates.
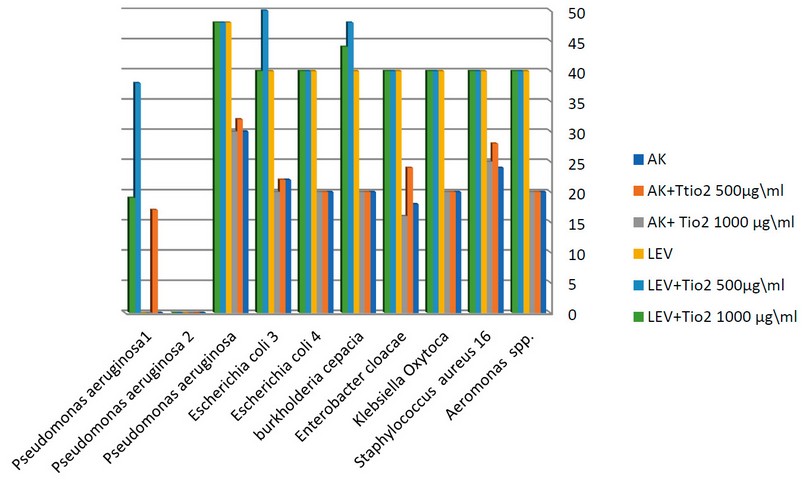
Figure 5. The synergistic effect of TiO2 NPs at a chosen concentration with antibiotics against environmental bacteria.
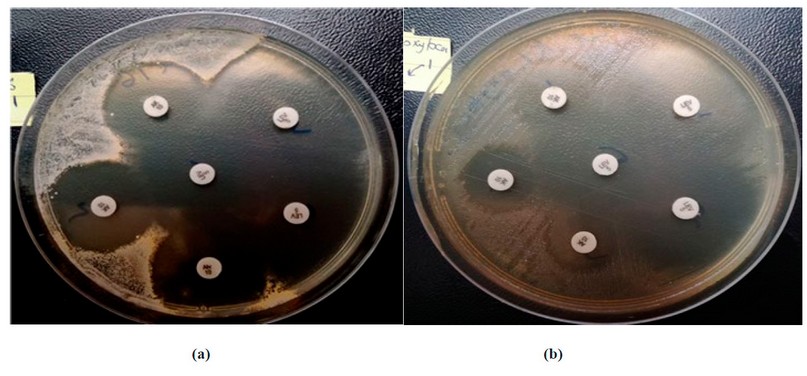
Figure 6. the inhibitory activity of the (Tio2).
DISCUSSION
Research has shown that the field of application of NPs in the medical field can increase the performance and effectiveness of therapeutic drugs, and mixing drugs with NPs leads to a process of accumulation in diseased tissues of the mixed substance. medicine23
Titanium dioxide (TiO2) inorganic nanoparticles have been used in the last decades because they may have a general mechanism of toxicity against bacteria24,9
According to several studies, it is believed that the metal oxides carry the positive charge while the microorganisms have negative charges; this causes electromagnetic attraction between microorganisms and the metal oxides, which leads to oxidization and, finally, death of microorganisms25 They cause pits or holes, on bacterial cell wall could be associated with internalized particles, leading to increased permeability and cell death26,27 ((In other words, the difference in electrical charges between polluted metals and living organisms causes a state of attraction between them, which leads to the accumulation of heavy metals in the body of the living organism and it dies.))TiO2 nanoparticles due to their small size and high surface to volume ratio undergo a higher level of interaction with the bacterial cells surface than the larger particles, resulting in a high antibacterial activity28
The result of this study may differ from other studies in the response of bacterial species towards TiO2 NPs at different concentrations because there is no photocatalytic or UV activation; also, the study was done by using the disk diffusion method, whereas many studies done in a liquid medium which may be favoring the close interaction between the suspended nanoparticles and the Gram-positive microbial cells, which could better attach and anchor to the surface of the microbial cells, causing structural changes and damages leading to cell
death29
TiO2 NPs can directly oxidize components of cell signaling pathways and even change gene expression by interfering with transcription factors. This result suggested that TiO2 NPs affect the microorganisms by not only oxidative damage but also bacteria aggregation and biofilm formation, which directly influenced pathogenicity30
CONCLUSIONS
Antibiotic treatment alone (or sometimes) fails to eradicate microbial infections like a medical device-related biofilm. Combining TiO2 NPs promising agent with antibiotics may be possible to eliminate s in the future.
Funding: Self-Funding.
Acknowledgments The author would like to thank Biology depart. , University of Mosul for supporting this research and Dr.Adeeba Younus shared her guidance.
Conflicts of Interest: The authors declare that they have no conflict of interest in this study.
REFERENCES
1.The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates
Martina Lorenzetti, Iztok Dogša,§ Tjaša Stošicki,§ David Stopar,§ Mitjan Kalin,∥ Spomenka Kobe, and Saša Novak ACS Appl. Mater. Interfaces 2015, 7, 1644−1651
2. A. Erdem • D. Metzler • D. Cha • C. P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light ,Huang 2015.
3. Visai L, De Nardo L, Punta C, Melone L, Cigada A, Imbriani M, Arciola CR. Titanium oxide antibacterial surfaces in biomedical devices. Int J Artif Organs,2011, 34: 929-946.
4. Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small,2011, 7: 1919-1931
5. Xie Y, Heo SH, Yoo SH, Ali G, Cho SO. Synthesis and photocatalytic activity of anatase TiO2 nanoparticles-coated carbon nanotubes. Nanoscale Res Lett,2009, 5: 603–607
6. Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed,2011, 6: 1463-1473
7. Yan M, Chen F, Zhang J, Anpo M. Preparation of controllable crystalline titania and study on the photocatalytic properties. J Phys Chem B,2005, 109:8673–8678
8. Banerjee AN. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: focus on TiO2-based nanostructures. Nanotechnol Sci Applicat,2011, 4: 35–65.
9. Natividad Castro-Alarcón1, José Luis Herrera-Arizmendi, Luis Alberto Marroquín-Carteño1, Iris Paola Guzmán-Guzmán, Armando Pérez-Centeno and Miguel Ángel Santana-Aranda. Antibacterial activity of titanium dioxide nanoparticles, intrinsic and doped with indium and iron.Microbiology Research International Vol. 4(4), pp. 55-62, November 2016.
10. M. Long, J. Wang, H. Zhuang, Y. Zhang, H. Wu, and J. Zhang, «Performance and mechanism of standard nano-TiO2 (P-25) in photocatalytic disinfection of foodborne microorganisms—Salmonella typhimurium and Listeria monocytogenes,» Food Control, vol. 39, no. 1, pp. 68–74, 2014.
11. K. Gupta, R. P. Singh, A. Pandey, and A. Pandey, «Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus.P. aeruginosa,» Beilsten Journal of Nanotechnology, vol. 4, no. 1, pp. 345–351, 2013.
12. S. Bonetta, S. Bonetta, F. Motta, A. Strini, and E. Carraro, «Photocatalytic bacterial inactivation by TiO2-coated surfaces,» AMB Express, vol. 3, no. 1, pp. 1–8, 2013.,
13. N. Yao and K. Lun Yeung, «Investigation of the performance of TiO2 photocatalytic coatings,» Chemical Engineering Journal, vol. 167, no. 1, pp. 13–21, 2011.].
14. Zhou, J. J., Wang, S. Y., & Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. Journal of food science,2009, 74(7), N50–N56.
15. L. Bastarrachea, S. Dhawan, and S. S. Sablani, «Engineering properties of polymeric-based antimicrobial films for food packaging,» Food Engineering Reviews, vol. 3, no. 2, pp. 79–93, 2011
16. Siti Hajar Othman, Nurul Raudhah Abd Salam, Norhazlizam Zainal, Roseliza Kadir Basha, Rosnita A. Talib, «Antimicrobial Activity of TiO2 Nanoparticle-Coated Film for Potential Food Packaging Applications», International Journal of Photoenergy, vol. 2014, Article ID 945930, 6 pages, 2014.
17. Thirunavukkarasu Santhoshkumar, Abdul Abdul Rahuman, Chidambaram Jayaseelan, Govindasamy Rajakumar, Sampath Marimuthu, Arivarasan Vishnu Kirthi, Kanayairam Velayutham, John Thomas, Jayachandran Venkatesan, Se-Kwon Kim. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties, Asian Pacific Journal of Tropical Medicine (2014)968-976
18. Ziental, D., Czarczynska-Goslinska, B., Mlynarczyk, D. T., Glowacka-Sobotta, A., Stanisz, B., Goslinski, T., & Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials (Basel, Switzerland),2020, 10(2), 387. https://doi.org/10.3390/nano10020387
19. Daniel Ziental, Beata Czarczynska-Goslinska, Dariusz T. Mlynarczyk, Arleta Glowacka-Sobotta, Beata Stanisz, Tomasz Goslinski and Lukasz Sobotta. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387
20. Ansari, M. A., Abdul, H. M., Joshi, G., Opii, W. O., & Butterfield, D. A. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. The Journal of nutritional biochemistry,2009, 20(4), 269–275.
21. Melvin P . Weinstein, MD. James S . Lewis II. Clinical and Laboratory Standards Performance Standards for Antimicrobial Susceptibility Testing,2020.
22. P. Nithya Devi1, J. Sathiyabama, S. Rajendran, R. Joseph Rathish and S. Santhana Prabha. Influence of malic acid-Zn2+ system on inhibition of corrosion of mild steel in simulated concrete pore solution prepared in well water,2017.
23. Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6.
24. Taylor, E., & Webster, T. J. Reducing infections through nanotechnology and nanoparticles. International Journal of Nanomedicine,2011, 6, 1463–1473.
25. Zhang H, Chen G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-podsol-gel method. Environ Sci Technol 2009;34(8):2905-10.
26. Ravishankar Rai, V. and Jamuna Bai, A. Nanoparticles and Their Potential Application as Antimicrobials, Science against Microbial Pathogens: Communicating Current Research and Technological Advances. In: Méndez-Vilas, A., Ed., Formatex, Microbiology Series,2011, No. 3, Vol. 1, Spain, 197-209;
27. Holt KB, Bard AJ. Interaction of silver (1) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy of micromolar Ag. Biochemistry 2005; 44(39): 13214-23 ).
28. Morteza Haghi , Mohammad Hekmatafshar Mohammad B. Janipour³, Saman Seyyed gholizadeh, Mohammad kazem Faraz, Farzad Sayyadifar, Marjan Ghaedi. ANTIBACTERIAL EFFECT OF TiO2 NANOPARTICLES ON PATHOGENIC STRAIN OF E. coli. International Journal of Advanced Biotechnology and Research ISSN 0976-2612, 2016.
29. Lane Pineda,1 Andre Chwalibog, ´ 1 Ewa Sawosz,2 Anna Hotowy,1 Jan Elnif,1 and Filip Sawosz1 . Investigating the Effect of In Ovo Injection of Silver Nanoparticles on Fat )Uptake and Development in Broiler and Layer Hatchlings. Hindawi Publishing Corporation Journal of Nanotechnology Volume 2012, Article ID 212486,p7.
30. Carol López de Dicastillo, Matias Guerrero Correa, Fernanda B. Martínez, Camilo Streitt and Maria José Galotto. Antimicrobial Effect of Titanium Dioxide Nanoparticles. Submitted: August 31st, 2019 Reviewed: December 18th, 2019 Published: January 27th, 2020 DOI: 10.5772/intechopen.90891
Received: October 23, 2022 / Accepted: January 15, 2023 / Published:15 February 2023
Citation: Hazem Najem A, Mahmood Khudhur I, Ali G M A. Inhibitory effect of Titanium dioxide (Tio2) nanoparticles and their synergistic activity with antibiotics in some types of bacteria. Revis Bionatura 2023;8 (1)34. http://dx.doi.org/10.21931/RB/2023.08.01.34






















