Vol 7 No 4 2022- 51

Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activity from avocado seed (Persea americana mill)
Dorely David1, Andrés Felipe Alzate2, Benjamín Rojano2, Ledys S. Copete-Pertuz3, Ricardo Echeverry4, Jhony Gutierrez4, Isabel Cristina Zapata-Vahos4[1]*
1 Tecnoparque Nodo Rionegro. Grupo de Investigación en Innovación y Agroindustria (GIIA). Centro de la Innovación La Agroindustria y la Aviación, Servicio Nacional de Aprendizaje – SENA, Vereda la Bodega-Zona Franca-Bodegas 14 y 15, CP 054040 Rionegro, Colombia. ddavid00@misena.edu.co,
2 Laboratorio Ciencia de los Alimentos, Facultad de Ciencias, Universidad Nacional de Colombia– Sede Medellín, Calle 59A No 63-20, CP 050034 Medellín, Colombia. afalzatea@unal.edu.co, brojano@unal.edu.co.
3 Compañía Nacional de Levaduras, Levapan S.A, Cr27 A 40-470, 763028 Valle del Cauca, Colombia lscopete@unal.edu.co.
4 Universidad Católica de Oriente- Facultad Ciencias de la Salud- Grupo de investigación APS.
*Corresponding author:izapata@uco.edu.co
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.51
ABSTRACT
The increase in the demand for Hass avocado has brought a rise in the generation of inedible waste such as peel and seed, by-products that are rich in bioactive substances. In the present study, aqueous, ethanolic, and supercritical fluid extracts were obtained from fresh seed and dry seed, which were analyzed to determine the antioxidant capacity measured through 2,2-diphenyl-2-picrylhydrazyl free radical (DPPH); 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC) methods as well as the content of phenolic compounds. In addition, the antimicrobial activity of strains of food interest, such as Listeria monocytogenes, Salmonella enterica Typhimurium and Escherichia coli was evaluated. The ethanolic extract of fresh seed presented the highest antioxidant and antimicrobial activity. The aqueous extract of fresh seed registered a significant antioxidant capacity but an absence of antimicrobial activity. In contrast, the ethanolic extract of dry seed showed a representative antimicrobial activity on both S. enterica Typhimurium and L. monocytogenes, but low antioxidant activity. E. coli exhibited resistance against all the assessed extracts. The results from this work highlight the opportunity to consider the Hass avocado seed extracts as a novel alternative to replace or reduce the use of synthetic antioxidant and antimicrobial additives in food.
Keywords: Waste by-product; Aqueous extract; Ethanolic extract; Supercritical extraction; Polyphenols; Free radical.
INTRODUCTION
Persea americana Miller (Lauraceae) is an evergreen tree native to Central America and cultivated in tropical and subtropical areas. Its cultivation is highly valued because it presents an edible fruit known as an avocado that can ripen even after being harvested1. There are several varieties of this fruit; the Hass variety is the most accepted one by consumers. It is estimated that between five and six million tons of avocados are harvested annually, which continues to grow due to increased demand2. Hass avocado has different organoleptic and nutritional qualities that differentiate it from other fruits, including a smooth texture and a pleasant flavor and color. It stands out for its high content of fat-soluble vitamins, phytosterols, proteins and monounsaturated fatty acids such as linoleic acid 3. These compounds have been widely related as beneficial for health against metabolic disorders such as hypercholesterolemia, arterial hypertension, diabetes and fatty liver disease 4 5.
An edible portion of the avocado is only a part of the whole fruit. It mainly corresponds to the pulp, consumed directly or used as the main ingredient for the production of guacamole or sauce or for the oil extraction that can be used in food, cosmetics or pharmaceutical preparations; the rest of the fruit is usually discarded or little used 6 7. Avocado residues are the peel and the seed that together represent between 30-33% of the total weight of the fruit, being the seed approximately 15 to 16%8. These by-products are currently considered a promising source of various bioactive compounds, among which polyphenolic combinations stand out, such as flavonoids, phenolic acids, and tannins 9 10.
Several epidemiological studies have shown that a regular intake of polyphenols, especially flavonoids, reduces the impact of chronic diseases such as diabetes, various types of cancer and cardiovascular and neurodegenerative diseases.11 12 The ability to trap free radicals generated in the course of these diseases is the mechanism that partly explains the contribution of these substances to a reduced occurrence of these pathologies 13. On the other hand, polyphenols are also used as natural antioxidants, helping to increase the shelf life of food and other consumer products 14. Likewise, many reports of antibacterial and antifungal capacity for these substances 15.
Significant amounts of procyanidins A and B have been reported in Hass avocado seed. 16 Also citric acid, hydroxytyrosol glucoside, caffeoylquinic acid, tyrosol glucoside, catechin and quercetin derivatives, and vanillic acid. A higher sterol content has also been reported in the seed extracts than in the pulp, which has also shown anti-inflammatory, anticarcinogenic and increased free radical scavenging potential 17.
Based on this, the present study aimed to obtain supercritical fluids extracts of Hass avocado seed, evaluating the antioxidant activities and antibacterial effects (against foodborne pathogens microorganisms present in food such as Listeria monocytogenes, Salmonella enterica Typhimurium and Escherichia coli)
MATERIALS AND METHODS
Plant material
The samples of avocado fruits (P. americana Mill. cv. Hass) were collected in Guarne (average temperature: 21 ºC and altitude: 2,150 m.a.s.l.) department of Antioquia, Colombia.
Once the material was pulped, the seeds were sanitized with an antibacterial solution of 0.3%v/v citrosan for 5 minutes. After the disinfection process, the material was cut into smaller pieces with a knife. Half of this material was ground and labeled FS, Fresh Seed. Another half of the seeds were dried at 50 °C for six hours and finely crushed in a cyclone-type laboratory mill (Udy Corporation, Colorado, USA); this material was labeled DS, Dry Seeds.
Extraction of bioactive compounds
Extraction with ethanol and water
Ethanolic and aqueous extracts were prepared separately using 10 g of seed powder (DS and FS) and 50 mL of each solvent. The mixtures were homogenized in ultraturrax at 9000 rpm for 5 min (IKA-Werk, Staufen, German) and centrifuged for 15 min at 5000 ×g. Subsequently, the supernatants were added in amber flasks, and 50 mL of the solvents were added to the precipitates again to homogenize and centrifuge them a second time. Finally, the two supernatants were mixed and stored at -20°C until use 18. Table 1 shows the identification and coding of the samples according to the extraction method used.
Extraction by supercritical fluid
Extraction was carried out in a Speed SFE Applied Separations equipment (Pennsylvania, USA) with a capacity of 100 mL. CO2 in the supercritical state was employed as extraction solvent using temperatures (40°C and 50°C) under pressures of 20 MPa and 30 MPa. In this experiment, 30 g of dried seeds in the avocado powder were taken and extracted for 40 min, and later the extract was stored in sealed test tubes at -20°C until the tests were carried out 19. Extraction capacity supercritical fluid was expressed in percentage. Assays were performed in triplicate.
Total polyphenol content
Polyphenol quantification was performed by the Folin-Ciocalteau colorimetric method, with some modifications 20. In test tubes, 50 µL of the sample, 125 µL of Folin-Ciocalteau reagent, 425 µL of sodium carbonate solution (7.1%), and water to complete 1000 µL were mixed. The reaction mixture was kept in the darkness for 60 min, and after this time, the absorbance was determined at 760 nm in a PG-Instruments spectrophotometer (Leicestershire, United Kingdom). A calibration curve was made using gallic acid as a standard. The results were expressed as equivalent gallic acid per 100 g sample (mg GAE/100 g).
Antioxidant capacity tests
DPPH free radical scavenging activity
The antioxidant activity of Hass avocado seeds was evaluated by the ability to trap the stable radical DPPH (2,2-diphenyl-2-picrylhydrazyl free radical), according to the methodology reported by Rojano (2011)21 with some modifications. In a test tube, 10 µL of sample and 990 µL of a DPPH solution (0.2 mM) were added. The exact amount of DPPH and 10 µL of the sample solvent were used as a reference. After 30 min of reaction, the absorbance at 517 nm was measured in a Multiskan Spectrum spectrophotometer (Thermo-Scientific, Waltham, MA, USA). The calibration curve was constructed using Trolox as a reference antioxidant, and the results were reported as equivalent μmol Trolox per 100 g of sample (μmol TE/100 g).
ABTS free radical scavenging activity
The antiradical ability of Hass avocado seeds is based on the discoloration of ABTS•+. The cationic radical ABTS•+ was generated by an oxidation reaction of ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6 ammonium sulfonate) with potassium persulfate according to the methodology described by Rojano (2011) 21. In the assay, 10 μL of sample and 990 μL of ABTS•+ solution were used; after 30 min of reaction, the change in absorbance with respect to a reference was determined at 734 nm. The reference consisted of a mixture of 990 µL of ABTS•+ radical solution and 10 µL of sample solvent. A calibration curve was constructed using Trolox as the reference antioxidant, and the results were reported as equivalent μmol Trolox per 100 g of sample (μmol TE/100 g).
Ferric reducing antioxidant power (FRAP) assay
FRAP methodology evaluates the ability of a sample to reduce the complex formed between iron and TPTZ (2,4,6-tripyridyl-s-triazine), where iron in its ferric form (Fe+3) becomes ferrous iron (Fe+2). This change can be measured spectrophotometrically 22. A 50 μL portion of the sample was mixed with 900 μL of a FRAP solution (1 mL of 10 mmol/L TPTZ and 1 mL of 20 mmol/L FeCl3 in 10 mL of pH 3.4 acetate buffer). The mixture was incubated for 30 min, and the absorbance was measured at 593 nm on a multiscan spectrum spectrophotometer (Thermo-Scientific). A standard curve was made using ascorbic acid as a reference. The results were expressed as equivalent mg of ascorbic acid per 100 g sample (mg AAE/100 g).
Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity – ORAC)
Experiments employed Trolox as a standard and controlled temperature and pH conditions (37 °C and 7.4, respectively). The assay was determined by diluting Trolox in 75 mM phosphate buffer (pH 7.4) and water-acetone (1:1, v/v) for ORAC-H (Hydrophilic) and in 7% β-methyl cyclodextrin for ORAC-L (Lipophilic). An excitation and emission wavelength of 493 nm and 515 nm, respectively, were used. 3 mL of the following mixture were prepared: 21 µL of a 10 mM fluorescein solution, 2899 µL of phosphate buffer, 50 µL of 600 mM AAPH, and 30 µL of the sample or 500 mM Trolox; as a control, the sample solvent was used. The antioxidant effect was calculated using the differences in areas under the fluorescence intensity decay curve between the negative control and the sample, and it was compared against the area under the Trolox curve 23. The results were expressed as equivalent Trolox values (μmol TE/100 g of sample), according to Equation 1.

Where AUC is the area under the curve corresponding to the sample, control or Trolox, and f is the concentration ratio between Trolox and the sample.
Antibacterial activity
The antimicrobial capacity of the extracts was determined using the good diffusion methodology for 3 strains of foodborne pathogenic bacteria, Escherichia coli (ATCC 25922), Salmonella enterica Typhimurium (ATCC 14028) and Listeria monocytogenes (ATCC 19118) at the first pass, according to the procedures described by Hudzicki 24. This methodology allows us to measure and compare the areas of inhibition of microbial growth of the extracts. The activation of the pathogenic microorganisms was carried out 24 h before the tests on the trypticase soy agar (TSA) method reported by Davidson and Parish 25. They were seeded by the streak method and incubated at 37 °C. After activation, the colonies were inoculated in Brain Heart Infusion (BHI), then the microorganisms were standardized on a scale of 0.5 Mac Farland; later, the solution was seeded on the surface Muller Hinton agar. Besides, four equidistant wells were made in the culture medium to obtain a circular well to the bottom of the Petri dishes. Then, randomly, 100 µL of each extract covered each well, and the Petri dishes were incubated for 24 h at 37 °C. Water and ethanol were used as negative controls (C-), and ciprofloxacin antibiotic (160 mg/mL) as positive control (C+). In addition, two Petri dishes were left with only the culture medium as environmental control. The results were reported as the inhibition halo diameter around the wells measured in millimeters (mm). All assays were performed in triplicate.
Statistical analysis
The results of the antioxidant capacity were analyzed using the analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests at 5% level of probability. The tests were carried out with the R Studio software version 3.5.0.
RESULTS AND DISCUSSION
Different Hass avocado seed extracts were obtained from some processes such as supercritical fluid extraction, as well as percolating and mechanical maceration with water and ethanol for both fresh seeds (FS) and for seeds that were dried at 50 ºC (DS) (Table 1). In total, 8 different extracts were analyzed to determine the presence and content of total polyphenols and phenolic acids; a complete antioxidant characterization was also carried out, and finally, the antibacterial capacity was presented on three of the main pathogenic bacteria. The results were promising and allowed further progress in using this by-product as a source of bioactive substances.

Table 1. Identification and coding of samples obtained by different extraction methods for Hass avocado seeds.
Extraction of bioactive substances
After performing the supercritical fluid extraction of the dried Hass avocado seeds (DS) under different pressure and temperature conditions, the capacity in the extract production was measured (Table 2). The highest extraction capacity (2.01%) was reached at 50 °C and 30 MPa pressure, followed by 50 °C and 20 MPa (1.63%), which indicates that a higher temperature substantially improves the extractive processes of this seed when this extraction methodology is used.

Table 2. Percentage of extraction capacity by supercritical fluid of dried Hass avocado seeds.
The extracts obtained presented lipophilic characteristics and, according to Daiuto et al. (2014)26. Hass avocado seed gives 3.32% lipids. However, these values may vary depending on the height and soil where the avocado is grown; this would explain why supercritical fluid extraction using CO2 as a solvent is very efficient in extracting lipid compounds. The extraction capacity results achieved in this research are comparable with those reported by Polania (2014)27, who obtained extractions capacities of 0.5% at 10 MPa and 60 °C, using ethyl acetate and 3% ethanol as cosolvent and capabilities of 3.6% with 6% methanol at 15 MPa and 50 °C.
Total polyphenol content
The Hass avocado seed extracts presented significant values of total polyphenols; the results are shown in Table 3. The extraction of fresh seeds (FS) with ethanol was the one that presented the best results (10.65 mg GAE/g), followed by aqueous extraction for FS (8.28 mg GAE/g); whereas the seeds dried (DS) had a polyphenol content much lower than FS. These results indicate that the drying of the sources, despite being at a relatively low temperature (50 ºC), caused significant degradation of this compound. Similar results have been reported by Segovia-Gomez et al. (2014) 28, who evaluated a process to optimize the extraction of polyphenols with different proportions of ethanol. The phenolic compounds in the seed extracts are of great importance since they are part of a group of secondary metabolites considered natural antioxidants with potential benefits for human health: anticancer activity, anti-inflammatory activity 17, and inhibition of gastric ulcer formation 29.
Antioxidant capacity
Considering that oxidation reactions are complex and that the bioactive substances present in Hass avocado seeds can exert their antioxidant action by different mechanisms, different antioxidant tests, based on the transfer of an electron (DPPH, FRAP and ABTS) and transfer of hydrogen atoms (ORAC), were carried out in this research to characterize the antioxidant potential of this by-product 30. The antioxidant capacity of the FS and DS extracts in water, and ethanol is presented in Table 3. The highest antioxidant activity was obtained for the ethanolic extract of fresh seed (FS-EtOH), reaching the highest values in the different antioxidant tests, followed by fresh seed extract with water (FS-H2O). On the other hand, the dry seed extracts in water (DS-H2O) and ethanol (DS-EtOH) showed lower values, demonstrating a low antioxidant capacity. From this, it is inferred that the high antioxidant capacity presented by the FS extracts is directly related to the higher content of total polyphenols presented by these samples and that the heat treatment showed a significant decrease in the antioxidant capacity.

Table 3. Antioxidant capacity and content of antioxidant metabolites of aqueous and ethanolic extracts of Hass avocado seeds.
The same letters per column mean no significant differences between extracts.
The results attained by FRAP showed that Hass avocado seed extracts present reducing substances that contribute to the total antioxidant capacity, especially FS-H2O and FS-EtOH, which have the highest values. Other authors reported similar behaviors of reducing power in ethanolic extracts from avocado seeds with values among 0.28 – 0.73 mg/mL FeSO4 31
The response of the DPPH free radical scavenging capacity was superior for FS compared to DS in water and ethanol. Some studies that have characterized the avocado found that the Hass variety contains greater antioxidant capacity than other avocado varieties, such as Fuerte. Wang and others (2010) evaluated the parts of the Hass avocado, finding 189.8 µmol TE/g FW in the peel and 164.6 µmol TE/g FW in the seed 6.
In ABTS assays, a similar behavior to DPPH and FRAP was evidenced, finding that the FS-EtOH sample presented the highest trapping of the cationic radical ABTS•+, followed by FS-H2O. In another investigation, values of 300 µmol TE/g DW were reported for avocado seeds 30, taller than those found in this work. Some authors have reported the presence of procyanidins, catechins, epicatechins, caffeoylquinic acid, vanillic acid, flavonoids, phenylpropanoids and tannins, among others, in by-products of avocado 32 33; compounds that contribute to the stabilization of DPPH and ABTS free radicals. Thus, the Hass avocado seed extracts presented a high reducing power and a remarkable antioxidant capacity by the methodologies used in this research.
Regarding the ability to trap oxygen free radicals (ORAC), this methodology was used in its two variants; the hydrophilic variant (ORAC-H) was performed on the extracts of FS and DS obtained in water and ethanol, and the lipophilic variant (ORAC-L) was used for the seed extracts which were attained by supercritical fluid. Results are summarized in Table 4.

Table 4. ORAC values of extracts obtained by supercritical fluid, water and ethanol from fresh and dried Hass avocado seeds.
The aqueous extracts had a greater capacity to trap the hydroxyl radical (52.23 µmol TE/ g sample and 51.47 µmol TE/ g sample for DS-H2O and FS-H2O, respectively) than the ethanolic extracts (10.62 µmol TE/ g sample and 14.75 µmol TE/ g sample for DS-EtOH and FS-EtOH, respectively), showing statistically significant differences. Regarding the lipophilic samples, no statistically significant differences were found, ORAC-L values were around 30 µmol TE/ g sample. The potential of these extracts to trap radicals is very important due to the harmful effect of free radicals in food and various biological systems 34.
Wang et al. (2010) 6 reported ORAC activity in various avocado varieties, finding 428.8 µmol equivalents of Trolox TE/g of fresh seed for the Hass variety. The authors found, for 7 types studied, that in the avocado seed, there is a remarkable antioxidant activity measured by DPPH and ORAC, in addition to the content of phenols and procyanidin, which were above the results indicated for the avocado skin and pulp. Another investigation reported ORAC activity of 310 µmol Trolox/g, dry weight 30. Soong and Barlow (2004) 35 reported that the content of secondary metabolites and the antioxidant capacity are higher in the seed than in the Hass avocado pulp.
Antibacterial capacity
Figure 1 illustrates the inhibition halos for S. Typhimurium (ATCC 14028), L. monocytogenes (ATCC 19118) and E. coli (ATCC 25922) facing the extracts evaluated. Significant differences (p-value <0.05) with respect to the positive control (ciprofloxacin, 160 mg/mL) were found. The FS-EtOH and DS-EtOH extracts presented a greater growth inhibition of L. monocytogenes and S. Typhimurium than the others. Ethanolic extracts were obtained from fresh and dry seeds, reaching an inhibition range similar to the positive control, with diameters of 38.16 mm and 26.94 mm for L. monocytogenes and 26.17 mm and 19.90 mm. for S. Typhimurium, respectively (Figure 1). Some studies attribute this activity to compounds such as phytosterols, triterpenes, fatty acids, furoic acids, flavonoids, polyphenols, and proanthocyanidins36. Raymond and Dykes (2010) 37 reported higher antimicrobial activity of ethanolic extracts than aqueous ones in Gram-positive and Gram-negative bacteria except for E. coli, with minimum inhibitory concentrations of 104.2 μg/mL for Salmonella enteritidis and 416.7 μg/mL for L. monocytogenes.
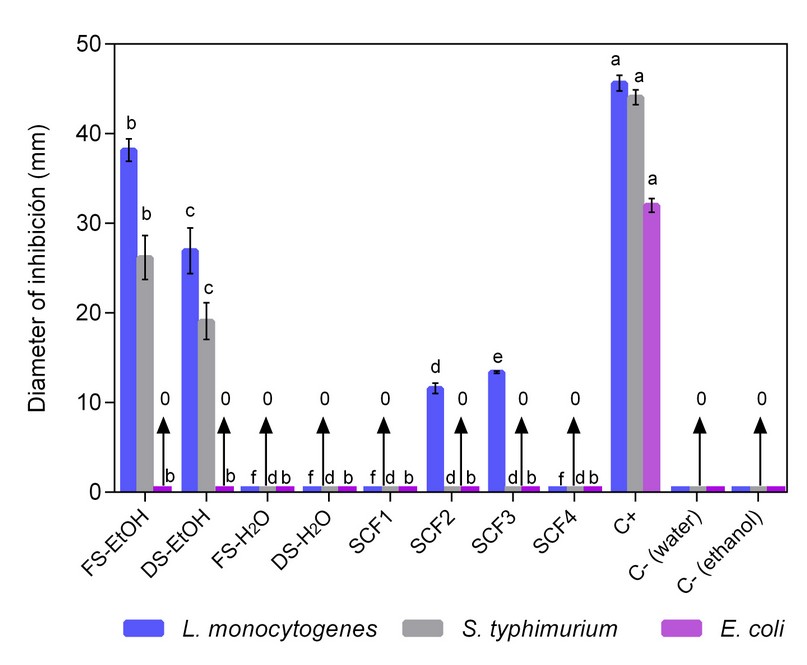
Figure 1. Antibacterial activity of FS-EtOH, DS-EtOH, FS-H2O, DS-H2O, SFC1, SFC2, SFC3, SFC4 avocado seed extracts, C+ and C- on L. monocytogenes, S. typhimurium and E. coli. The same letters per column mean that there are no significant differences.
The FSC2 and FSC3 extracts showed growth inhibition of L. monocytogenes, whereas the other extracts evaluated did not show an antimicrobial effect. E. coli presented resistance against all assessed extracts, and both positive and negative (water and ethanol) control behaved according to expectations (Figure 1). The results obtained for E. coli agree with results previously reported by Hennessey (2019)38, who found resistance from Staphylococcus aureus subsp. ATCC 29213 and E. coli against Lorena variety avocado seed extracts extracted with solutions of sodium hydroxide, ethanol, and water; while Romaní et al. (2017)39 found that the ethyl acetate fraction of the seeds of Persea americana Mill, Hass variety, presented phenolic compounds with antibacterial activity at a concentration of 10% facing the E. coli strain with a minimum inhibitory concentration of 0.625 mg/mL. Rodríguez et al. (2011)40 evaluated the antimicrobial activity of the seed, skin and pulp of two varieties of avocado on E. coli CECT 4267. The authors found antimicrobial activity for the Fuerte variety and resistance facing the Hass variety extract, which suggests dependence of the antimicrobial response to the plant variety and the type of solvent used in the extraction processes.
CONCLUSIONS
The seeds generated as by-products of avocado industrialization are an interesting source of extracts with essential concentrations of polyphenols and antimicrobial potential. In this work, different extracts were obtained in various solvents, and the best results of antioxidant and antimicrobial capacity were for the ethanolic extract of fresh seed (FS-EtOH), being very effective in the growth inhibition of S. Typhimurium and L. monocytogenes microorganisms. The aqueous extract of fresh seed (FS-H2O) also had a great antioxidant capacity, although it did not show any inhibitory effect on the bacteria evaluated. The dry seed ethanolic extract (DS-EtOH) showed significant antimicrobial activity on S. typhimurium and L. monocytogenes, but low antioxidant activity. With these results, natural Hass avocado seed extracts can be considered a good alternative in the food industry to replace or reduce the use of antioxidant additives and synthetic antimicrobial agents.
ACKNOWLEDGEMENTS
The authors would like to thank Servicio Nacional de Aprendizaje -SENA (Tecnoparque nodo Rionegro) and the Universidad Católica de Oriente (UCO) for the financial support of this research.
REFERENCES
1. Araújo RG, Rodriguez-Jasso RM, Ruiz HA, Pintado MME, Aguilar CN. Avocado by-products: Nutritional and functional properties. Trends Food Sci Technol. 2018;80(August):51–60.
2. Tremocoldi MA, Rosalen PL, Franchin M, Massarioli AP, Denny C, Daiuto ÉR, et al. Exploration of avocado by-products as natural sources of bioactive compounds. Vol. 13, PLoS ONE. 2018.
3. Tan CX. Virgin avocado oil: An emerging source of functional fruit oil. J Funct Foods. 2019;54(26):381–92.
4. Del Toro-Equihua M, Velasco-Rodríguez R, López-Ascencio R, Vásquez C. Effect of an avocado oil-enhanced diet (Persea americana) on sucrose-induced insulin resistance in Wistar rats. J Food Drug Anal. 2016 Apr 1;24(2):350–7.
5. Furlan CPB, Valle SC, Östman E, Maróstica MR, Tovar J. Inclusion of Hass avocado-oil improves postprandial metabolic responses to a hypercaloric-hyperlipidic meal in overweight subjects. J Funct Foods. 2017 Nov 1;38:349–54.
6. Wang W, Bostic TR, Gu L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010;122(4):1193–8.
7. González-Fernández JJ, Galea Z, Álvarez JM, Hormaza JI, López R. Evaluation of composition and performance of composts derived from guacamole production residues. J Environ Manage. 2015 Jan 1;147:132–9.
8. Chil-Núñez I, Molina-Bertrán S, Ortiz-Zamora L, Dutok CMS, JOUR. Estado del Arte de la especie Persea americana Mill (aguacate) ; State of the Art of the specie Persea americana Mill (avocado) ; Estado da Arte da espécie Persea americana Mill (abacate). 2019;(July). Available from: https://www.amazoniainvestiga.info/index.php/amazonia/article/view/49
9. Zaki SAEH, Ismail FAEA, Abdelatif SH, El-Mohsen NRA, Helmy SA. Bioactive compounds and antioxidant activities of avocado peels and seeds. Pakistan J Biol Sci. 2020;23(3):345–50.
10. Dabas D, Elias RJ, Ziegler GR, Lambert JD. In Vitro Antioxidant and Cancer Inhibitory Activity of a Colored Avocado Seed Extract. Int J Food Sci. 2019;2019(2017).
11. Sharma P, Hajam YA, Kumar R, Rai S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomedicine Plus [Internet]. 2022;2(1):100188. Available from: https://doi.org/10.1016/j.phyplu.2021.100188
12. de Carvalho JTG, Da Silva Baldivia D, de Castro DTH, dos Santos HF, dos Santos CM, Oliveira AS, et al. The immunoregulatory function of polyphenols: implications in cancer immunity. J Nutr Biochem [Internet]. 2020;85:108428. Available from: https://doi.org/10.1016/j.jnutbio.2020.108428
13. Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J Funct Foods. 2015 Oct 1;18:820–97.
14. Lima GPP, Vianello F, Corrêa CR, Campos RA da S, Borguini MG. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr Sci. 2014;05(11):1065–82.
15. Carrillo-Tomalá C, Díaz-Torres, Raúl-Guerra-Guamán ARS. Actividad antimicrobiana de extractos hidroalcohólicos de hojas de dos variedades de Antimicrobial activity of hydroalcoholic extracts of leaves of two varieties of Mangifera Indica L . Rev Cienc UNEMI. 2020;13:69–77.
16. Jimenez P, Garcia P, Quitral V, Vasquez K, Parra-Ruiz C, Reyes-Farias M, et al. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev Int [Internet]. 2021;37(6):619–55. Available from: https://doi.org/10.1080/87559129.2020.1717520
17. Alkhalaf MI, Alansari WS, Ibrahim EA, ELhalwagy MEA. Antioxidant, anti-inflammatory and anticancer activities of avocado (Persea americana) fruit and seed extract. J King Saud Univ – Sci. 2019;31(4):1358–62.
18. Rojas JJ, García AM, López AJ. Evaluación de dos metodologías para determinar la actividad antimicrobiana de plantas medicinales. Boletín Latinoam y del Caribe Plantas Med y Aromáticas. 2005;4(2):28–32.
19. Reyes-Najar A, Castro-Vargas HI, Rodríguez-Varela LI, Quijano-Celis CE, Parada-Alfonso F. OBTENCIÓN DE EXTRACTOS DE JENGIBRE (Zingiber officinale) EMPLEANDO CO2 SUPERCRÍTICO. Rev la Acad Colomb Ciencias Exactas, Físicas y Nat [Internet]. 2011 [cited 2021 Nov 26];35(136):381–5. Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0370-39082011000300011&lng=en&nrm=iso&tlng=es
20. Zapata-Vahos IC, Rojas-Rodas F, David D, Gutierrez-Monsalve JA, Castro-Restrepo D. Comparison of antioxidant contents of green and red leaf lettuce cultivated in hydroponic systems in greenhouses and conventional soil cultivation. Rev Fac Nac Agron Medellin. 2020;73(1).
21. Alberto Rojano B, Cristina Zapata Vahos I, Felipe Alzate Arbeláez A, Juleza Mosquera Martínez A, Bernardo Cortés Correa F, Gamboa Carvajal L. Polifenoles y Actividad Antioxidante del Fruto Liofilizado de Palma Naidi (Açai Colombiano) (Euterpe oleracea Mart) Polyphenols and Antioxidant Activity of the Freeze-Dried Palm Naidi (Colombian Açai) (Euterpe oleracea Mart). 2011.
22. Benzie, I. F., & Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;16(18):209–19.
23. Rautenbach F, Venter I. Hydrophilic and lipophilic antioxidant capacity of commonly consumed South African fruits, vegetables, grains, legumes, fats/oils and beverages. J Food Compos Anal. 2010 Nov 1;23(7):753–61.
24. Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. 2009;
25. Davidson P., Parish M. Methods for testing the efficacy of food antimicrobials. Food Technol. 1989;43:148–155.
26. Daiuto ÉR, Tremocoldi MA, Matias De Alencar S, Vieites RL, Minarelli PH. Chemical composition and antioxidant activity of the pulp, peel and by products of avocado ‘hass. Chem Compos Antioxid Act pulp, peel by Prod avocado ‘hass. 2014;36(2):417–24.
27. Polania Barreto W. Actividad antioxidante de residuos del aguacate Hass (Persea americana Mill. var Hass) sometidos a extracciones clásicas y a fluidos presurizados. Univ Nac Colomb Tesis. 2014;
28. Gómez FS, Peirósánchez S, Iradi MGG, Azman NAM, Almajano MP. Avocado seeds: Extraction optimization and possible use as antioxidant in food. Antioxidants. 2014;3(2):439–54.
29. Athaydes BR, Alves GM, Assis ALEM de, Gomes JVD, Rodrigues RP, Campagnaro BP, et al. Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res Int [Internet]. 2019;119(May 2018):751–60. Available from: https://doi.org/10.1016/j.foodres.2018.10.057
30. Figueroa JG, Borrás-Linares I, Lozano-Sánchez J, Segura-Carretero A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res Int [Internet]. 2018;105(November 2017):752–63. Available from: https://doi.org/10.1016/j.foodres.2017.11.082
31. Folasade OA, Aderibigbe Olaide R, Olufemi TA. Antioxidant Properties of Persea Americana M. Seed As Affected By Different Extraction Solvent. Orig Res Artic J Adv Food Sci Technol [Internet]. 2016;3(2):101–6. Available from: https://www.researchgate.net/publication/301552950
32. López-Cobo A, Gómez-Caravaca AM, Pasini F, Caboni MF, Segura-Carretero A, Fernández-Gutiérrez A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT. 2016 Nov 1;73:505–13.
33. Agnieszka Kosińska, Magdalena Karamać, Isabel Estrella, Teresa Hernández, Begoña Bartolomé GAD. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varietiesNo Title. J Agric Food Chem. 2012;60(18):4613–9.
34. Turner S, Davicino R, Alonso R, Ferraro G, Filip R, Anesini C. Potential use of low-NDGA Larrea divaricata extracts as antioxidant in foods. Rev Peru Biol. 2011;18(2):159–64.
35. Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88(3):411–7.
36. Cabrera J., L. Dilas. & PM. Determinación de la actividad antioxidante y antimicrobiana del extracto etanolico de la semilla de Persea Americana Miller Var.Hass “Palta”. Rev Perspect. 2015;13(32):69–77.
37. Chia D, RaymondA. TW, Gary. Antimicrobial activity of crude epicarp and seedextracts from mature avocado fruit (Persea Americana) of three cultivars. Pharm Biol. 2010;48(7):753–756.
38. Hennessey-Ramos L, Murillo-Arango W, Guayabo GT. Evaluation of a colorant and oil extracted from avocado waste as functional components of a liquid soap formulation. Rev Fac Nac Agron Medellin. 2019;72(2):8855–62.
39. Romaní L, Enciso E, Cárdenas V, Condorhuamán YM. Artículo original Actividad antibacteriana de compuestos fenólicos de semillas de. Cienc Invest. 2017;20(2):19–22.
40. Rodríguez-Carpena JG, Morcuende D, Andrade MJ, Kylli P, Estévez M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem. 2011;50(10):5625–35.
Received: December 25, 2021 / Accepted: June 12, 2022 / Published:15 November 2022
Citation: David D, Alzate A F, Rojano B, Copete-Pertuz L S, Jhony Gutierrez R E, Zapata-Vahos I C. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activity from avocado seed (Persea americana mill). Revis Bionatura 2022;7(4) 51. http://dx.doi.org/10.21931/RB/2022.07.04.51



















