Vol 7 No 4 2022- 17

Nutritional values and phytochemical analysis of Allium calocephalum Wendelbo, a valuable endemic wild garlic to Zagros mountains
Wajed I. Hussein1, Hadar S. Faizy2, Sami M.A. Youssef 3&4
1 Department of Forestry, College of Agricultural Engineering Sciences, University of Duhok, Ministry of Higher education and scientific research, Kurdistan Region Government KRG, Iraq.
2 Department of Recreation and Ecotourism College of Agricultural Engineering Sciences, University of Duhok, Ministry of Higher education and scientific research, Kurdistan Region Government KRG, Iraq.
3 AMAP (botany and Modelling of Plant Architecture and vegetation), University of Montpellier / CIRAD / CNRS / INRA / IRD – AMAP, CIRAD TA A51/PS2, 34398 Montpellier Cedex 5, France.
4 Department of Recreation and Ecotourism, College of Agricultural Engineering Sciences, University of Duhok, Ministry of Higher education and scientific research, Kurdistan Region Government KRG, Iraq.
* Correspondence: hadar.said@uod.ac; Tel.: (009647508872179)
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.17
ABSTRACT
Wild edible plants provide the local people with food and medicines and are considered one of the natural ecosystem services. These wild edible diets and herbal medicine always reflect local communities’ regional identity and their traditional ecological knowledge. In the new global economy, the natural product field has become a central issue for preserving the traditional culture related to nature, particularly in the context of a sustainable environment. This research study aims to determine the nutritional value and phytochemical contents in a wild population of Allium calocephalum. This wild edible garlic, endemic to the Zagros mountains, is overharvested by Kurdish rural people to enhance their food security at a household level and to perpetuate the preservation of their natural heritage. Here, we estimated the total phenols, flavonoids, carbohydrates, protein, fibers, ash, oil yield, and significant mineral content in both leaves and bulbs of A. calocephalum. Phytochemical analyses were conducted at the Faculty of Agricultural Engineering Sciences (University of Duhok) and the environmental directory of Duhok, Kurdistan Region of Iraq, to get an overview of its nutrients and phytochemical values.
Interestingly, a high level of phenolic compounds was obtained from bulbs (0.684 mg gallic acid equivalents/g of dry extract, eq.100g-1). The lowest level of phenolic compounds was found in leaves (0.522 mg gallic acid equivalents/g of dry extract, eq.100g-1). Simultaneously, the bulbs extract gave higher content of flavonoid compounds than the leaves extract (6.31 and 4.73 μg quercetin equivalents/g of dry extract, eq.100g-1, respectively, for the bulbs and leaves). The highest dry weight basis of total carbohydrates, energy value (Kcal), oil content, and moisture content were observed in bulbous parts, and the values were 71.75, 408.86 (Kcal), 9.52, and 92.37, respectively. On the other side, the highest dry weight basis of total protein, fibers, and Ash content was observed in shoot parts, and the values were 15.93, 13.89, and 9.32, respectively. The evidence from this research study supports the idea that this Zagrosian endemic wild garlic enhances the food security and the nutrient diet values of the rural Kurdish people.
Keywords: Wild garlic; ethnobotany; edible plants; food security; natural resources; herbal medicine.
INTRODUCTION
Our knowledge of environmental conservation and human well-being is fundamentally shaped by the linkages and interactions between human cultures and plants 1-3. The production of food, shelter, clothes, transportation, fertilizers, flavors and scents, and medicines have all been basic human needs that have always been met by nature 4-6. In this sense, sophisticated traditional medical systems that have existed for thousands of years and continue to offer humanity novel treatments have their roots in wild edible plants (WEPs) 7,8. Despite some claims that plants possess therapeutic properties turning out to be incorrect, medicinal plant therapy is founded on actual data from hundreds, if not thousands, of years of use 8,9. The practice of herbal medicine has gained credibility and acceptability within the local and medical communities in recent years, and there has been an increase in interest in the field 9,10. The search for possible chemotherapeutic agents in nature continues, and over the past 40 years, numerous powerful medications have been produced from wild plants 7.
In addition to their therapeutic properties, these WEPs have significant nutritional value and are a crucial component of the diets of rural communities worldwide. These WEPs offer the human body high dietary benefits, including vitamins (A, B, C, etc.), a good number of minerals, carbohydrates, protein, fat, antioxidants, etc. 11,12. They have actively aided in ensuring food security, particularly during times of crisis 13. Due to their few side effects, the wide variety of options, and high nutritional value, local communities tend to turn to natural resources for treatment and regular foods 2,11. Any portion of the plant with active ingredients, including the roots, bulbs, stems, bark, leaves, flowers, fruits, seeds, bulbs, gum, etc., can be used to make these wild foods or wild edible diets 14. The Allium genus serves as a well-known illustration of these WEP usages. The nutritional value of many Allium taxa has been examined globally 15, as well as their ethnobotanical aspect 16-18 and phytochemistry 19,20. For illustration, in traditional medicine, the Allium taxa are widely used for treating common health problems like wounds, bacterial infections, and worms 21,22.
On the other hand, current research in contemporary medicine has demonstrated the healing properties of their active ingredients, including their ability to fight cancer 23, anti‐inflammatory, cardio-protective 24, hepato-protective 25, antithrombotic 26, etc. Furthermore, the phytochemical characteristics of Allium taxa include essential oil, steroidal sapogenins, saponins, tannins, carbohydrates, proteins, vitamins, flavonoid glycosides, organic acids, amino acids, fatty acids, alkaloids, etc., have been well-studied 19,20,27,28. In addition, from the perspective of natural services, it is expected that many significant new treatments will be found and made commercially available in the future, just as they have up until now, by following the cues given by traditional knowledge and experiences.
People in daily life have used wild plants since the beginning of civilization 6. These wild plants provide a significant portion of the natural ecosystem services regarded as edible for human use (i.e., food and dishes) 29,30. The regional identity of the indigenous populations and their traditional ecological knowledge is always reflected in these wild edible diets 31-33. The traditional wild food knowledge has been documented in numerous nations, regions, and continents around the world and has occasionally been referred to as a diet for local/regional communities: In Europe 33, Mediterranean Region 34,35, Iran 36, Turkey 18,37, Kurdistan Region of Iraq 16,38-40 etc. As a result, it is getting harder to disregard the significance of the WEPs in modern human society in the context of a sustainable environment. The market for natural products has emerged as a critical concern in the new global economy for maintaining the traditional culture associated with nature. Even though wild edible plants have always played a key role in local and regional folk traditions, they are gradually losing position regarding plant usage patterns and behaviors 41,42. The expansion of the agroindustry, urbanization, changes in lifestyle and land use decrease and/or loss of the local traditional ecological knowledge, and decreased interaction with nature are the key factors contributing to the changes in wild edible plant use patterns 31,33.
Additionally, some native plant species may be threatened by the current ongoing overharvesting and growing demand for edible wild plants by local people in urban areas. Therefore, it is essential to implement the appropriate modifications to traditional cultural traditions to help the local populace comprehend the significance of preserving the WEPs and their natural habitat. Additionally, wild edible plants are being included in both modern and traditional innovation models for local healthy diets and food security. Therefore, one of the most important justifications for protecting natural ecosystem resources is the potential for unknown pharmacological properties. This research study’s objective was to offer information on the nutritional value and phytochemical components of wild edible garlic, Allium calocephalum Wendelbo, from Liliaceae family. This study has provided a broad view of its potential traditional use in current wild diets from the perspective of ethnobotany. Wild garlic from this particular Allium species is endemic to Zagros Mountains in NE Iraq and SE Turkey 18,43. It is primarily found between 1200 and 2500 meters above sea level in the Mateen, Gara, Bradost, Hindreen, Sherîne, and Pîrês mountains among oak trees and scrub. Traditional uses include eating its leaves and young stem, frequently fried or combined with other dishes 16,18. However, little is known about this wild edible garlic, and no studies have been done on its nutritional benefits or phytochemical properties.
MATERIALS AND METHODS
Species study
Allium calocephalum is endemic wild garlic to the Zagros mountains of NE Iraq and SE Turkey 43. It typically occurs in the upper forest zone of mountains like the Mateen, Gara, Bradost, Hindreen, Sherîne, and Pîrês ranges. Kurdish rural communities refer to it as «Soriyaz» locally. This garlic is typically collected from the wild between early spring and summer, frequently sold in traditional Kurdish markets 16,18. Due to the increasing demand brought on by the rising human population, this wild edible garlic is severely threatened in this context of overharvesting. The ecology of seed germination and seedling growth of A. calocephalum species have recently undergone extensive research from the standpoint of natural regeneration 44. From the perspective of biological conservation, it has been suggested that ethno-domesticating and cultivating this species will help prevent the removal of it from its natural habitats by giving many small farmers in rural areas a source of income 44.
Plant Material collection
Fresh plant material, including leaves and bulbs of the A. calocephalum species of Zagrosian endemic garlic, was taken from the north side of Gara Mountain (Hariké Village, Deralok District; latitude: 37.007705°; longitude: 43.689504°; elevation 1240 m). To estimate the phytochemical components and nutritional value correctly, We took the plant material in April 2018 when the species was in its complete development cycle. The harvested plant materials were placed in a polyethylene bag to protect against moisture loss while being transported in the field. Taxonomically, the plant species were identified using the Flora of Iraq 43 as a source. Various specimens were deposited in the herbarium of the University of Duhok’s Forestry Department (DPUH), College of Agricultural Engineering Sciences.
Sample Preparation
The shoot materials (leaves with stems) and bulbs were carefully cleaned by cutting the bulb roots and removing their outer tunics. After this cleaning procedure, each plant material was washed under running water and placed in the plastic basket to drain the remaining moisture. The samples were then split into smaller pieces and shade dried at room temperature for around 20 days. The dried parts were then pulverized using an electric household blender. For additional analysis, the powdered samples were kept in a glass jar and refrigerated (approximately 5 °C). The laboratory facility at the College of Agricultural Engineering Sciences, University of Duhok, was used to estimate the various sample parameters, including moisture content, total ash, crude oil, crude fiber, crude protein, energy value (kcal), and total carbohydrate on a dry weight basis, using 100g of a dried powdered sample.
Determination of Total Moisture Content
According to a technique provided in 45 Method No. 44-15A, the moisture content was measured: A. calocephalum fresh samples weighing 3 g each (for both leaves and bulbs) were put into a pre-weighed crucible (W0) and weighed (W1) before being heated in a forced draft oven for 24 hours at 98–100 °C. The crucibles were taken out, let to cool in a desiccator, and then weighed. The procedure was performed several times until the crucible, and wild garlic sample had a constant weight (W2). This was done to guarantee that the crucibles were dry. The equation below was used to determine moisture content:
%Moisture =(w1-w2)/(w1-w0)×100
Where:
W0 = Weight (g) of the empty crucible
W1 = Weight (g) of fresh garlic sample + empty crucible
W2 = Weight (g) of dried sample + empty crucible
Determination of Crude Proteins (Kjeldahl nitrogen method)
According to Kjeldahl’s procedure, which included protein digestion, distillation, and titration 46, the dried powdered sample was examined for crude protein concentration.
Protein Digestion: By combining 0.5 g of each bulb and leaf sample with 10 ml of concentrated H2SO4 (98% conc.) and adding 2 ml of perchloride acid (70%) before heating, the protein content was ascertained. The mixture was then heated in the digestion chamber until transparent residue contents were obtained. Then it was given time to cool. The digest was cooled before diluting it with the proper amount of distilled water to make it 100 ml.
Protein Distillation
The Markham distillation equipment was first steamed for 15 minutes. The condenser was then positioned beneath a 50 ml conical flask containing 5 ml of 2% boric acid to submerge the condenser tip in the solution. Through a tiny funnel hole, 5.0 ml of the digested material was pipetted into the apparatus’s main body. After washing the digest with distilled water, 5 ml of (10 N) NaOH solution was added. The condenser’s digest was boiled until enough ammonium salt was obtained. The green color of the boric acid plus indicator solution indicated that all of the ammonia released had been captured.
Protein Titration: By determining how much HCl was used in the process, the concentration of 0.01 N HCl was added to the solution in the receiving flask until it changed color to red. Following titration, the formula was used to determine the % nitrogen:
%N=(V.HCl*N HCl(0.01)*Nitrogen Atomic Mass(14))/1000*(100ml D.Water)/(5ml Sample Solution)*(%100 )/(0.5 Wt.of Sample)
Where, %N = Nitrogen percentage
V. HCl = Volume (ml) of acid required to titrate
N HCl = Normality of the HCl
D. Water = Distilled water added
Wt.= Weight of a sample (g).
The formula was then used to determine the sample’s crude protein content as a percentage.:
%Crude Protein =%N*F (6.25)
Where F= Conversion factor is equivalent to (6.25)
Determination of Crude Oil
Soxhlet extraction for six hours was used to evaluate the crude fat content of the powdered sample. Approximately 3.0 g of samples were precisely weighed and placed in labeled thimbles. 100 ml of hexane 96% was added to the dried boiling flasks (250 ml), which had been considered in accordance. Cotton wool was used to cover the extraction thimbles securely. After putting the Soxhlet apparatus together, the flask was attached to the extractor, set on the heating mantle, and allowed to reflux for 6 hours. After the extraction was finished, the thimble was taken off, and the boiling flask was heated in a hot air oven at 70 ºC until the solvent evaporated and the hexane was almost eliminated. The flask was dried, cooled in a desiccator, and weighed again 45.
The percentage of crude oil was calculated using the formula:
Crude oil (%) = (Wt. of oil (g) / Wt. of the original sample (g)) × 100
Determination of Crude Fiber
The percentage of crude fiber was calculated using the literature-recommended method of 47. Following this, 2 g of a dried, fat-free sample of A. calocephalum powder was weighed (W0) into a 1000 ml conical flask. H2SO4 (20 ml with 20%) and distilled water (100 ml) were combined, and the mixture was gently heated for 30 minutes. The content was filtered through Whatman No.1 filter paper. A spatula was used to scrape the residue back into the flask. After that, 100 ml of distilled water and 20 ml of 10% NaOH were added, and the mixture was allowed to boil gently for 30 minutes. The contents were filtered, and the residue was extensively treated with hot distilled water, 10% HCl once, ethanol twice, and petroleum ether three times. It was allowed to dry, scraped into the crucible, and then dried in an oven for an additional night at 105 ºC. Following removal, the sample was cooled in a desiccator. The sample was weighed (W1), then ashed in a muffle furnace for 90 minutes at 600 ºC, removed, cooled in a desiccator, and weighed once again (W2). The percentage of crude fiber was calculated using the equation:
%Crude Fiber=(w1-w2)/w0×100
Where:
W0 = Weight of sample (g)
W1 = Weight of dried sample (g)
W2 = Weight of ash sample (g)
Determination of Ash Content
Ash is the inorganic residue that is left after material has either been wholly burned at a high temperature of 550 °C in a muffle furnace or completely oxidized. It is the totality of all non-volatile inorganic elements. The ash content determination was followed by a method according to 48. In this experiment, 2 g of the dry powder sample was weighed (W1) into pre-weighed empty porcelain crucibles (W0), where it was then burned for 5 hours at 55 °C in an ash-muffling furnace to produce ash. Afterward, the ash crucibles were removed, cooled in a desiccator, and reweighed (W2).
The % ash content was calculated as follows:
%Ash=(w2-w0)/(w1-w0)×100
Where:
W0 = Weight of empty crucible (g)
W1 =Weight of crucible + powdered sample (g)
W2 = Weight of crucible + ash sample (g)
Determination of Total Carbohydrate
The total percentage carbohydrate content at dry weight bases (DWB) in the A. calocephalum sample was followed by method 48. As a result, the carbohydrate content was determined by subtracting 100 from the difference between 100 g of the dry mass of a sample and the total values of its crude protein, crude fat, natural fiber, and ash constituents 46,49,50. The following equation estimated the total carbohydrate value:
%Carbohydrate (DWB)=100-( % crude fiber + % protein + % lipid + % ash)
Determination of Energy Value of Samples
Following the method of 51,52, the energy value (kcal/100 g) of the dried samples was calculated by multiplying the values of carbohydrate content by 4, protein content by 4, and fat content by 9. This equation estimated the energy value (kcal/100 g):
Energy Value = (Crude protein × 4) + (Total carbohydrate × 4) + (Crude fat × 9)
Minerals Analysis
To determine P, K, Ca, Mg, S, Fe, Mn, Zn, Cu, Cd, Pb, Al, and Cr, plant material was digested in a di-acid solution (wet digestion). HNO3 and HClO4 were used in a 9:4 ratio for the procedure. A 100 ml volumetric flask containing 1.0 g of the dried sample powder was filled with 10 ml of nitric acid (HNO3) and left overnight for pre-digestion. Add 8 ml of HClO4 the following day and gently stir with a magnetic stirrer. The flask was put on a hot plate and heated to a temperature of about 100 °C. After that, it was heated to a temperature of about 260 °C for more than an hour to cause the red NO2 vapors to disappear. The volume of the flask’s contents was reduced to 3 to 4 ml through continued evaporation without drying up the mixture. The digestion process continued until the solution color altered and became colorless. The flask was filled with 20 ml of deionized water after cooling. Deionized water was used to make up the volume and dilute it to the proper concentration, while Whatman No.1 filter paper was used to filter the sample. The prepared solution was used for the determination of K, Ca, Mg, S, Fe, Mn, Zn, Cu, Cd, Pb, Al, and Cr. Atomic absorption spectroscopy (AAS) was used to estimate these mineral nutrients. While phosphorus (P) assessment was performed using a spectrophotometer using a colorimetric method, with the absorbance being measured at 882 nm 52-54. To measure Na in A. calocephalum, the sample was prepared using the dry ashing method. After digestion, sodium (Na) was estimated using flame photometry 46.
Total phenolics and Flavonoids content analysis
Sample preparation and extraction
Extracted crude plant material, which was prepared by combining the dried powder with 500 ml of 96% ethanol in a conical flask, was utilized to estimate the total phenolic and flavonoid content. This extraction process took roughly 24 hours. Then, plant extracts were filtered through layers of folded filter paper, and ethanol extracts were concentrated using a Rotary Vacuum Evaporator at 40 °C. The solvent was eliminated using a rotary evaporator. This method involved carefully controlling the solvent removal while using a vacuum. The water bath was heated to 40 °C while the distillation flask was rotated between 150 and 200 rpm with the distillation flask filled to 50% with ethanol plant extracts. Following the evaporation of ethanol, the condensed crude plant material was collected and scraped off the wall of the distillate flask using a spatula. The remaining plant stock was scraped off the flask’s wall using a small amount of ethanol solvent, and it was added together before being allowed to evaporate at room temperature overnight. The obtained crude plant material weight was then recorded and stored in a freezer at a temperature of -5 °C for further analysis.
Total phenol content (TPC)
Folin-Ciocalteu’s method was used to determine the total phenolic content in the ethanolic extract of A. calocephalum according to 55,56. Folin-Ciocalteu’s phenol reagent and concentrated extract material solution were mixed in a test tube with 1 mL each. The mixture was then left in the dark for about 5 minutes, at which 10 ml of a 7% sodium carbonate (Na2CO3) solution was added, followed by 13 ml of deionized distilled water, and the mixture was gently shaken until well mixed. The mixture was left in the dark for about 30 minutes at room temperature (20–23 °C) to allow the reaction to complete. Then the absorbance of the mixture’s blue color was determined at 760 nm using a UV spectrophotometer. The total phenol content was calculated using a gallic acid solution standard curve. The results were represented as milligrams of gallic acid equivalents (mg GAE/100 g) of extracted dried samples. The estimation of the total phenolic compounds in triplicate was performed.
Total flavonoid content (TFC) determination
Total flavonoid content was determined according to the method described by 57. Consequently, a half milliliter of each extract (0.1 gm/ml) in methanol was mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, then 0.1 ml of 1 M potassium acetate, and 2.8 ml of distilled water were added. The combination then stayed at room temperature for 30 minutes. A UV/visible spectrophotometer was used to detect the reaction mixture’s absorbance at 415 nm. Every sample was tested twice. Following the process for all traditional quercetin solutions (12.5–100 μg /ml), a standard curve was created in methanol. Results were stated as milligrams of quercetin equivalent per gram (mg QE/g) of extract.
RESULTS
According to the study’s findings in Figure 1, the number of phenolic compounds was significantly influenced by the plant part used. Interestingly, bulbous sections showed a high level of phenolic compounds (0.684 mg gallic acid equivalents/g of dry extract, eq.100g-1); While the shoot sections, including the leaves and young stems, had the lowest concentration of phenolic compounds (0.522 mg gallic acid equivalents/g of dry extract, eq.100g-1). These findings strongly suggested that the portion used impacted the number of phenolic compounds overall.

Figure 1. Compare phenolic compounds for plant materials of Allium calocephalum of the shoot and bulbous parts.
The total flavonoids of Allium calocephalum extracts were estimated using the aluminum chloride colorimetric method Figure 2. The number of flavonoids in the bulbous parts extract was higher than that in the shoot parts extract the values were (6.31 and 4.73 μg quercetin equivalents/g of dry extract, eq.100g-1) respectively, for both bulbous and shoot parts.

Figure 2. Comparison of flavonoid compounds in plant materials of Allium calocephalum of the shoot and bulbous parts.
The results of proximate composition in studied sample materials are given in Table 1; the comparative analysis of the two portions of Allium calocephalum was obtained. The bulbous parts had the most significant dry weight basis of total carbohydrates (71.75%), while the shoot parts had the lowest percentage (54.33%). There was a substantial difference between the oil content of the two parts, with bulbous legs having a more excellent value (9.52%) than shoot parts (6.53%), and moisture content was highest in bulbous parts of Allium calocephalum (92.56%) as compared to shoot details (90.24%). Also, the highest energy value (Kcal) found in bulbous parts (408.86 Kcal) was comparable with the ( 339.84 Kcal) value found in shoot parts. Contrarily, the crude protein, crude fiber, and natural ash content were higher in the shoot sections, with discounts of 15.93%, 13.89%, and 9.32%, respectively. In contrast, the bulbous areas had the lowest values, at 9.05%, 6.84%, and 2.83 %, respectively.

Table 1. Proximate composition of Allium calocephalum plant in both shoot and bulbous parts.
The results in Table 2 showed that there was some variation in the macro-mineral contents of the two plant parts for calcium, phosphorus, magnesium, and sulfur; the shoot parts had the highest contents (384.82, 329.59, 154.549, and 349.8 mg/100g) respectively, as opposed to the bulbous parts (309.66, 46.35, 107.692, and 284.5 mg/100g). However, the bulbous sections had the highest potassium and sodium content (1995.902 and 372.402 mg/100g), respectively, while the shoot parts had the lowest potassium and sodium content (1343.6 and 159.6 mg/100g), respectively.
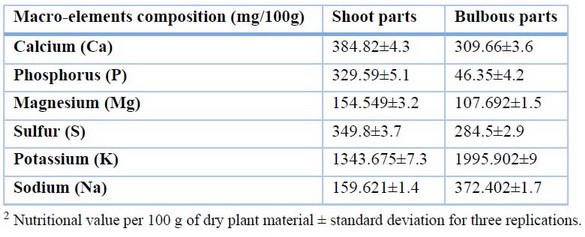
Table 2. Macro-mineral elements of the Allium calocephalum plant were studied in both shoot and bulbous parts.
The data in Table 3 show no significant changes between the micro-mineral contents analyses in the two parts of the plant. According to the study, the high concentrations of zinc, iron, manganese, lead, aluminum, and chromium identified in shoot parts were (3.857, 11.514, 0.714, 2.131, and 13.846 mg/100g) respectively, while the low concentrations detected in bulbous parts were (3.108, 3.5, ND, 1.668, 8.869, and 0.1 mg/100g). Unexpectedly, it was discovered that bulbous portions (0.8 mg/100g) had a higher level of copper content than shoot parts (0.582 mg/100g).
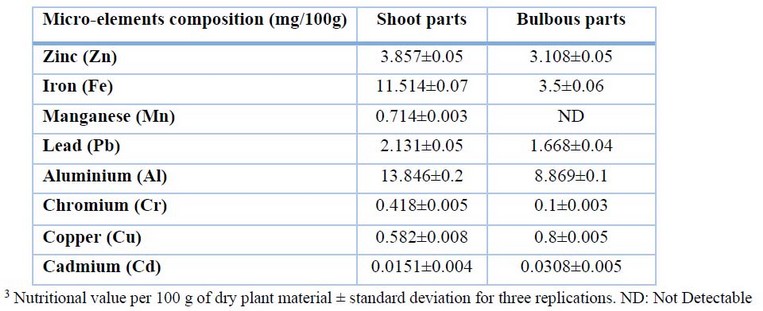
Table 3. Micro-mineral composition of Allium calocephalum plant in both shoot and bulbous parts.
Overall, differences in the chemical composition of Allium calocephalum Wendelbo and other plant tissues may be caused by various influences, including soil composition, growing conditions, temperature, precipitation, sun exposure, and interactions with other plants and animals in the ecosystem. Wide intraspecific variability was found mainly in carbohydrates, which are closely involved in plant metabolism. Hence, slight variations in the growth stage can lead to differences in carbohydrate and mineral element content, which are strongly influenced by environmental circumstances, including soil composition, among other aspects 74.
DISCUSSION
Previous research has highlighted the significance of the Allium taxa in terms of its pharmacological and nutritional properties 15,58. The indigenous communities have historically harvested these low-toxic edible vegetable plant species in large quantities for their daily diets, excellent flavor (which is rich in sulfur content), and medicinal properties (anticancer, antioxidant, antimicrobial, etc.) 19,20. Due to its numerous medical applications, which have a wide range of benefits against ailments like cancer, arthritis, and asthma, these natural phytochemical compounds are becoming more common in today’s society. In contrast to pharmaceutical chemicals, these natural phytochemicals are human-friendly medications since they can treat diseases without harming or endangering humans 59. The metabolic products are known as polyphones, and phenolic compounds are found in plant-based meals. These compounds had numerous biological and pharmacological properties that could offer protection from chronic illness 60. They are more active antioxidants than vitamins. They can neutralize oxidative free radicals 61.
The results of phenolic compounds are consistent with 62 observed for all tested cultivated garlic Allium sativum L extracts. Various levels of phenolics (0.05–0.98 mg gallic acid equivalents/g of dry extract). Also, agreement with 63 when they found that the total phenolic content in cultivated garlic Allium sativum L. varied from 3.4 mg gallic acid equivalents (GAE)/g of dry matter (dm) to 10.8 mg GAE/g of dm with a mean value of 6.5 mg GAE/g of dm in the bulbous parts. The average total phenolics and flavonoids of the wild leek are 5.77 mg GAE/g extract, and 0.86 64 reported entire phenolic contents for Allium porrum (0.369 mg GAE/g extract).
On the other hand, the results of total flavonoids coincide with those reported 65 when evaluating the phenolic and flavonoid contents and the antibacterial and antioxidant properties of onion (Allium cepa) and garlic (Allium sativum). The maximum value of total flavonoids was 17.64 mg EC/100 g, obtained from an 80% ethanolic garlic extract. The lowest value was 0.41 mg EC/100 g for aqueous oak extracts. According to studies in 66, the phenolic components of Allium sativum were lower (0.56 mg EC/g extract), while Allium cepa had higher levels of flavonoids (1.31 mg EC/g extract). The antioxidant activity of garlic was strongly correlated with its phenol content, which had antioxidant properties and flavonoid content. Phenol content, antioxidant activity and differences among garlic, onion and Allium calocephalum cultivars would facilitate the choice of cultivars with medicinal advantages 67.
These results of the dry weight basis of total carbohydrates, energy, oil content, the moisture content in bulbous parts dry weight basis of complete protein, fibers and ash that were observed agreed with the outcomes were consistent with those 68. It revealed a moisture content of about 78%, which was between the cultivated leek Allium porrum (86%) and garlic Allium sativum (64%). 69 found that wild leek has an average value of (4.23%), making it an attractive source of dietary fiber. According to the Food Nutrition Board’s recommendations, a 100 g portion can give (20.29%) of the daily amount needed for women and (11.21%) of the required daily amount for men. Additionally, compared to cultivated species, wild leeks had a more meaningful average fiber content (2.9%). Regarding protein content, A. sativum and A. porrum have (0.9 g/100 g and 2.1 g/100 g), respectively, but the average values found in wild leeks are (1.67 g/100 g) 68. 70 found that Allium sativa had the highest carbohydrate composition (16.60 g/100 g), but the wild Allium cepa had a higher carbohydrate composition (16.60 g/100 g). The primary macronutrient of the bulb has an energy value of (78.92 kcal/100 g) in Allium sativum, while Allium sativum and Allium porrum have an energy value of (139 kcal/100 g) 68. On the other side, the findings of macro-elements composition are consistent with those of 71, who reports that Chrysanthemum coronarium had a K content of roughly 1800 mg/100g, which was comparable to other culinary plants like savory, black thyme, and oregano, with values of about (1366, 1654.6, and 1962.5 mg/100g) respectively 72. Additionally, Capsicum’s Ca, P, and Mg contents (633, 148.5, and 443.2 mg/100g), respectively, were comparable to those of Reseda alba, which has average Ca, P, and Mg contents of about (1210, 250, and 220 mg/100g) respectively 71.
The results of the micro-elements composition showed that, contrary to expectations, there was no substantial difference between the two parts of the plant in the comparable cadmium concentrations. This result agrees with 73 findings, which indicated that the Zn contents of Zea mays L. and Ipomoea batatas (L.) Lam. were (3.8 and 3 mg/100 g), respectively. Additionally, 72 noted that cumin, caraway, and fennel each had a Fe concentration of (12.9, 4.67, and 9.72 mg/100g) respectively. It is widely known that the presence of some calcium-binding compounds, such as oxalic acid, which encourages the production of insoluble calcium oxalates and may be present in high concentrations in leafy vegetables, interferes with the absorption of calcium of plant origin.
The highest secondary metabolite contents, such as total phenolic and flavonoids, were found in the aerial parts and in plants collected from high-altitude areas. These results confirm the effects of different geographical and environmental regions66. The plants that are collected from high altitudes contain high secondary metabolites.
Environmental conditions are important factors affecting plant growth and chemical compound levels 68, reported photoperiod ( light and day lengths) affected plants content, essential oils and mineral nutrients71. Other studies have also noted the influence of altitude, drought and light intensity on plants’ growth and their contents 73; this confirmed the differences in the chemical composition affected by various altitudes and geographical positions. The researchers concluded that the ecological factors of habitat, such as altitude and soil physiochemical properties, could affect plant vegetative growth and change the quality and quantity of essential oils and chemical compounds in aromatic and medicinal plants. These changes happened as resistance to environmental stress 70, 72.
CONCLUSIONS
This research study has given an account of the reason for the traditional uses of Allium calocephalum by the rural communities in the Kurdistan Region of Iraq to enhance their food security and preserve their natural heritage. The results of this investigation show that this zagrosian wild endemic garlic has excellent nutritional value and phytochemical prosperities and is considered one of the natural ecosystem services. Interestingly, the phytochemical compound levels were significantly affected by the plant parts. Indeed, the highest level of phenolic and flavonoid compounds, total carbohydrates, energy value, and fat content were obtained from bulbous parts. In contrast, total protein, fibers, and Ash content were observed in shoot parts. Concerning the macro-mineral contents analysis (Calcium, Phosphorus, Magnesium, Sulphur, potassium, and sodium), both bulbs and leaves details showed variation in content level. The current findings add substantially to our understanding of the nutritional value and phytochemical prosperities of this wild edible garlic and will serve as a base for future ethnobotanical and pharmacological studies.
REFERENCES
1. Jones V. The nature and scope of ethnobotany. Vol 6. Chronica Botanica1941.
2. Balick MJ, Cox PA. Plants, people, and culture: the science of ethnobotany: Scientific American Library; 1996.
3. Millennium ecosystem assessment M. Ecosystems and human well-being. Vol 5: Island press Washington, DC; 2005.
4. Cragg GM, Newman DJ. A tale of two tumor targets: topoisomerase I and tubulin. The Wall and Wani contribution to cancer chemotherapy. Journal of Natural Products. 2004;67(2):232-244.
5. Minnis PE. People and plants in ancient western North America: University of Arizona Press; 2003.
6. Nesbitt M. Grains. The cultural history of plants: Routledge; 2012:50-65.
7. Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Molecular aspects of medicine. 2006;27(1):1-93.
8. Heinrich M, Williamson EM, Gibbons S, Barnes J, Prieto-Garcia J. Fundamentals of pharmacognosy and phytotherapy E-BOOK: Elsevier Health Sciences; 2017.
9. Hamilton A, Shengji P, Kessy J, Khan AA, Lagos-Witte S, Shinwari ZK. The purposes and teaching of applied ethnobotany. Vol 11: Citeseer; 2003.
10. Samuelsson G. Drugs of natural origin: a textbook of pharmacognosy, 5th Swedish Pharmaceutical Press. Stockholm, Sweden. 2004.
11. Geissler C, Powers HJ. Human nutrition: Oxford University Press; 2017.
12. Paul S. Mineral and Trace Elements. Human nutrition. Eleventh Edition, Elsevier Churchill Livingstone, Netherlands. 2006.
13. Tomkins M, Yousef S, Adam-Bradford A, et al. Cultivating refuge: The role of urban agriculture amongst refugees and forced migrants in the Kurdistan region of Iraq. Urban Agriculture and City Sustainability. 2019;131.
14. Cotton CM. Ethnobotany: principles and applications: John Wiley & Sons; 1996.
15. Demirci Kayiran S, Eroglu Ozkan E, Mataraci Kara E, Yilmaz MA, Zengin G, Boga M. Comprehensive analysis of an uninvestigated wild edible medicinal garlic species from Turkey: Allium macrochaetum Boiss. & Hausskn. Journal of food biochemistry. 2019;43(7):e12928.
16. Youssef S, Mahmood, A., Hussein, W., Véla, E. Montagnes du Zagros, un paradis terrestre aux pratiques ethnobotaniques vivantes. 2017;120:41-45.
17. Youssef S, Galalaey A, Mahmood A, Mahdi H, Véla E. Wild orchids of the Kurdistan Region areas: a scientific window on the unexpected nature of the North-Western Zagros2019.
18. FIRAT M. The ethnobotanical usage of some East Anatolian (Turkey) Allium L. species. Manas Journal of Agriculture Veterinary and Life Sciences. 2015;5(1):80-86.
19. García-Herrera P, Morales P, Fernández-Ruiz V, et al. Nutrients, phytochemicals and antioxidant activity in wild populations of Allium ampeloprasum L., a valuable underutilized vegetable. Food research international. 2014;62:272-279.
20. Panahandeh J, Farhadi N, Motallebi Azar A, Alizadeh Salteh S. Evaluation of Persian shallot (Allium hirtifolium) ecotypes for phytochemical components and antioxidant activity. Journal of Medicinal plants and By-product. 2016;5(2):217-226.
21. ÖZKAN GAM, KOYUNCU MA. Tradional medicinal plants used in Pınarbaşı area (Kayseri-Turkey). Turkish Journal of Pharmaceutical Sciences. 2005;2(2):63-82.
22. Lanzotti V, Scala F, Bonanomi G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochemistry Reviews. 2014;13(4):769-791.
23. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anticancer agent. Current cancer drug targets. 2003;3(1):67-81.
24. Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutrition journal. 2002;1(1):1-14.
25. Park SY, Je JY, Ahn CB. Phenolic Composition and Hepatoprotective Activities of A llium Hookeri Against Hydrogen‐Peroxide‐Induced Oxidative Stress in Cultured Hepatocytes. Journal of Food Biochemistry. 2016;40(3):284-293.
26. Ariga T, Seki T. Antithrombotic and anticancer effects of garlic‐derived sulfur compounds: A review. Biofactors. 2006;26(2):93-103.
27. Hu G, Mao R, Ma Z. A new steroidal saponin from the seeds of Allium tuberosum. Food chemistry. 2009;113(4):1066-1068.
28. Oku S, Ueno K, Tsuruta Y, et al. Sugar accumulation and activities of enzymes involved in fructan dynamics from seedling to bulb formation in onion (Allium cepa L.). Scientia Horticulturae. 2019;247:147-155.
29. De Groot RS, Fisher B, Christie M, et al. Integrating the ecological and economic dimensions in biodiversity and ecosystem service valuation. The economics of ecosystems and biodiversity (TEEB): ecological and economic foundations: Earthscan, Routledge; 2010:9-40.
30. Maes J, Paracchini ML, Zulian G. A European assessment of the provision of ecosystem services. Towards and atlas of ecosystem services. Ispra: Joint Research Centre, IES. 2011.
31. Alexiades MN. Ethnobotany in the Third Millennium: expectations and unresolved issues. Delpinoa. 2003;45(1):15-28.
32. Gerique A. An introduction to ethnoecology and ethnobotany: Theory and methods. Integrative assessment and planning methods for sustainable agroforestry in humid and semiarid regions. Advanced Scientific Training. Loja. 2006.
33. Luczaj L, Pieroni A, Tardío J, et al. Wild food plant use in 21 st century Europe, the disapperance of old traditions and the search for new ciusines involving wild edibles. Acta societatis botanicorum poloniae. 2012;81(4).
34. Leonti M, Nebel S, Rivera D, Heinrich M. Wild gathered food plants in the European Mediterranean: a comparative analysis. Economic botany. 2006;60(2):130-142.
35. Hadjichambis AC, Paraskeva-Hadjichambi D, Della A, et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. International Journal of Food Sciences and Nutrition. 2008;59(5):383-414.
36. Ghasemi PA, Momeni M, Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. African Journal of Traditional, Complementary and Alternative Medicines. 2013;10(2):368-385.
37. Ozbucak TB, Kutbay HG, Akcın OE. The Contributıon of Wıld Edible Plants to Human Nutrıtion in the Black Sea Regıon of Turkey. Ethnobotanical Leaflets. 2006;2006(1):10.
38. Ahmad SA, Askari AA. Ethnobotany of the Hawraman region of Kurdistan Iraq. Harvard papers in botany. 2015;20(1):85-89.
39. Ahmed HM. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. Journal of ethnobiology and ethnomedicine. 2016;12(1):1-17.
40. Mati E, de Boer H. Ethnobotany and trade of medicinal plants in the Qaysari Market, Kurdish Autonomous Region, Iraq. Journal of Ethnopharmacology. 2011;133(2):490-510.
41. Łuczaj Ł. Changes in the utilization of wild green vegetables in Poland since the 19th century: a comparison of four ethnobotanical surveys. Journal of ethnopharmacology. 2010;128(2):395-404.
42. Sõukand R, Kalle R. Change in medical plant use in Estonian ethnomedicine: a historical comparison between 1888 and 1994. Journal of Ethnopharmacology. 2011;135(2):251-260.
43. Townsend C, Guest G. Flora of Iraq, vol. 8: 128. Ministry of Agriculture & Agrarian Reform, Baghdad. 1985:137-177.
44. HUSSEIN WI, YOUSSEF SM. A COMPARATIVE STUDY OF SEED GERMINATION AND SEEDLING EMERGENCE OF TWO WILD EDIBLE ALLIUM SPECIES ENDEMIC TO ZAGROS AREAS. Journal of Duhok University. 2019;22(2):160-176.
45. Committee AAoCCAM. Approved methods of the American association of cereal chemists. Vol 1: Amer Assn of Cereal Chemists; 2000.
46. AOAC. Official methods of analysis: Aoac Washington, DC; 1990:1-50.
47. Udo E, Oguwele J. Laboratory Manual for Analysis of Soil planted and Water samples 3rd Ed. Department of Crop Production. University of Ilorin Kwara State, Nigeria. 1986.
48. James CS. Analytical Chemistry of Food. Chapman and Hall, London1995.
49. Sarkiyayi S, Agar T. Comparative analysis on the nutritional and anti-nutritional contents of the sweet and bitter cassava varieties. Advance journal of food science and technology. 2010;2(6):328-334.
50. McDonald P, Edwards, R. A. and Greenhalgh, J. F. D. Animal Nutrition. Singapore: Singapore Publisher plc. Ltd.; 1994.
51. Okwu DE, Morah FN. Mineral and nutritive value of Dennettia tripetala fruits. Fruits. 2004;59(6):437-442.
52. Hussain J, Rehman NU, Khan AL, Hamayun M, Hussain SM, Shinwari ZK. Proximate and essential nutrients evaluation of selected vegetables species from Kohat region, Pakistan. Pak. J. bot. 2010;42(4):2847-2855.
53. Khan I, Ali J, Tullah H. Heavy metals determination in medicinal plant Withania somnifera growing in various areas of peshawar, NWFP, Pakistan. Journal-Chemical Society of Pakistan. 2008;30(1):69.
54. Hussain J, Khan AL, Rehman N, Zainullah KF, Hussain ST, Shinwari ZK. Proximate and nutrient investigations of selected medicinal plants species of Pakistan. Pakistan Journal of Nutrition. 2009;8(5):620-624.
55. Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in–vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pacific journal of tropical biomedicine. 2013;3(8):623-627.
56. Khadabadi S, Deore S, Baviskar B. Experimental phytopharmacognosy. Nirali prakashan, page. 2011(47).
57. Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC complementary and alternative medicine. 2012;12(1):1-12.
58. Al-Snafi AE. Pharmacological effects of Allium species grown in Iraq. An overview. International Journal of Pharmaceutical and health care Research. 2013;1(4):132-147.
59. Banu KS, Cathrine L. General techniques involved in phytochemical analysis. International journal of advanced research in chemical science. 2015;2(4):25-32.
60. Kumar H, Kim I-S, More SV, Kim B-W, Choi D-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Natural product reports. 2014;31(1):109-139.
61. Tepe B, Sokmen M, Akpulat HA, Sokmen A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chemistry. 2006;95(2):200-204.
62. Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food chemistry. 2008;111(4):925-929.
63. Beato VM, Orgaz F, Mansilla F, Montaño A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods for Human Nutrition. 2011;66(3):218-223.
64. Tsouvaltzis P, Gerasopoulos D, Siomos A. Effects of base removal and heat treatment on visual and nutritional quality of minimally processed leeks. Postharvest Biology and Technology. 2007;43(1):158-164.
65. Abdul Qadir M, Shahzadi SK, Bashir A, Munir A, Shahzad S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. International journal of analytical chemistry. 2017;2017.
66. Gorinstein S, Park, Y.S., Heo, B.G., Namiesnik, J., Leontowicz, H., & Leontowicz, M. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. European Journal of Food Research and Technology. 2009(228):903–911.
67. Soto V, Gonzalez RE, Sance MM, Galmarini CR. Organosulfur and phenolic content of garlic (Allium sativum L.) and onion (Allium cepa L.) and its relationship with antioxidant activity. Paper presented at: VII International Symposium on Edible Alliaceae 11432015.
68. Souci SW, Fachmann W, Kraut H. Food composition and nutrition tables: Medpharm GmbH Scientific Publishers; 2000.
69. Trumbo P, Schlicker S, Yates A, Poos M. Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621-1630.
70. García Herrera P. Plantas silvestres de consumo tradicional en España: caracterización de su valor nutricional y estimación de su actividad antifúngica. 2014.
71. Akrout A, El Jani H, Zammouri T, Mighri H, Neffati M. Phytochemical screening and mineral contents of annual plants growing wild in the southern of Tunisia. Journal of Phytology. 2010;2(1):034-040.
72. Özcan M. Mineral contents of some plants used as condiments in Turkey. Food chemistry. 2004;84(3):437-440.
73. Olaofe O, Sanni C. Mineral contents of agricultural products. Food Chemistry. 1988;30(1):73-77.
74. Bernaert N, De Paepe D, Bouten C, et al. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food chemistry. 2012;134(2):669-677.
Received: 20 July 2022 / Accepted: 15 October 2022 / Published:15 November 2022
Citation: Hussein W I, Faizy H S, Youssef S M A. Nutritional values and phytochemical analysis of Allium calocephalum Wendelbo, a valuable endemic wild garlic to Zagros mountains. Revis Bionatura 2022;7(4) 17. http://dx.doi.org/10.21931/RB/2022.07.04.17



















