Vol 7 No 2 2022- 59

Nasma I. Al-Mamary1, Haitham L. Al-Hayali2,
1Ministry of Health/ Nineveh Health Department /Makhmour Health Sector. Iraq
2Department of Biology/ College of Science/ University of Mosul
*Correspondence: nasma.scp33@student.uomosul.edu.iq
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.59
ABSTRACT
This study aims to find safe and effective anti-leishmaniasis drugs; thus, the synergism between thalidomide and liposomal amphotericin-B was tested as antileishmanial on L. tropica and L. donovani promastigote in vitro. IC50, IC90 were determined at the Log phase of thalidomide and were (10), (25) µg/ml for L. tropica and (12.5), (30( µg/ml for L. donovani, Moreover IC50, IC90 were determined at Log phase of Liposomal amphotericin-B and were (5), (20) µg/ml for L. tropica and (5), (25) µg/ml for L. donovani. Additionally, synergistic effect IC50 of the two drugs were determined when Liposomal amphotericin-B fixed it, and thalidomide concentrations changed was (2.5+0.5) µg/ml on L. tropica and (2.5+1) µg/ml on L. donovan. When thalidomide was fixed, and Liposomal amphotericin-B was changed, it was (2.5+2) µg/ml for both L. tropica and L.donovani. The synergistic effect on the morphology of both promastigotes forms was observed.
Keywords. Leishmaniasis, thalidomide, liposomal amphotericin-B, synergistic.
INTRODUCTION
Leishmaniasis is a zoonotic parasitic disease caused by the Leishmania parasite. Leishmaniasis occurring in most tropical and sub-tropical countries is classified as a neglected tropical disease1. Leishmaniasis is an intracellular parasite transmitted by the bit of sand fly species phlebotomist. Leishmaniasis considers one of the seven most crucial tropical diseases on all continents2. Approximately 98 species are suspected vectors of human leishmaniasis3. More than 20 species of obligate intracellular protozoa of Leishmania caused human infection4. Limiting access to the treatment caused infection of more than one billion persons5. According to the classification of WHO, there are three types of Leishmaniasis infected the human:
Visceral Leishmaniasis (VL)
It is a fatal disease; more than 95% of the case if untreated directly. Human infection occurs due to (L. donovani, L. infantum) known as L. chagasi in the new world. The protozoan infects the phagocytic cell (macrophage and monocytes), amastigote transformed by macrophages to the liver, spleen and bone marrow. It caused swelling in them6.
Cutaneous Leishmaniasis (CL)
It is a common form of Leishmaniasis and causes skin lesions. This typically evolves from papules to nodular plagues to ulcerative sores. In old-world include L. major and L. tropica while L. mexicana, L. braziliensis, L. amazonesis, L. venezuelensis and L. ethiopica found in the new world7.
Mucocutaneous Leishmaniasis (MCL)
This type of Leishmania causes disfiguring lesions of the nose, mouth and throat mucous membranes. Infection can spread through the bloodstream or lymphatic system caused by L. braziliensis and L. panamensis. 8. The leishmaniasis treatment requires a very long time of therapy, is very limited, costly to the patients, and has high toxicity for most drugs 9. Pentavalent antimonials are the most commonly used drugs for treating Leishmaniasis, despite their variable efficacy for both cutaneous and visceral leishmaniasis 10. Due to its accumulation in the tissues, antimony can cause severe effects like vomiting, anorexia, nausea, myalgia, headache, abdominal pain, lethargy, and arthralgia; additionally, it rarely causes a severe reaction with cardiac arrhythmia11. Pentamidine and amphotericin B are the second-line drugs for Leishmaniasis. However, toxicity
And emerging resistance has discontinued the pentamidine uses, whereas amphotericin-B is capable of causing severe toxicity, hospitalization requirements, and the high costs of therapy12. Miltefosine is registered as an anticancer agent; with regards to CL and VL therapy, the main advantages were oral efficacy and a short course of treatment; therefore, it has significant limitations in the leishmaniasis therapy due to its teratogenic effects and longevity, which may enhance the resistance to drugs13.
Thalidomide (Thalomid) is a synthetic of glutamic acid (alpha- N- phthalimido-glutarimide) is a glutamic acid 14. The chemical structure is C13H10N2O4 with a 258.23 g/mol molecular weight. It is very efficacious in treating patients with chronic erythema nodosum leprae. Thalidomide works as selectively inhibiting tumor necrosis factor-α gene expression, which is concerned with nerve damage’s pathogenicity in leprosy15. Additionally, liposomal amphotericin B (AmBisome) is aliped-complex, colloidal and liposomal form; the composition consists of a mixture of phosphatidylcholine, cholesterol and distearoyl phosphatidylglycerol. Its structure is C47H73NO17, with a molecular weight of 924.09 g/mol. Amphotericin B is an antifungal agent effective versus clinically pertinent molds and yeasts containing Aspergillus, Candida spp. and filamentous molds, approved to treat fungal infections worldwide 16. The drug was developed to reduce adverse effects and improve pharmaCokinetics, bioavailability and lower toxicity but still very expensive and unstable
at higher temperatures 11. The study tackled the inhibitory effect of thalidomide and liposomal amphotericin B separately and synergistically on L. tropica and L. donovani promastigote and tried to reduce the dose of amphotericin in the presence of thalidomide. That combined therapy may be promising for the treatment of Leishmaniasis.
at higher temperatures 11. The study tackled the inhibitory effect of thalidomide and liposomal amphotericin B separately and synergistically on L. tropica and L. donovani promastigote and tried to reduce the dose of amphotericin in the presence of thalidomide. That combined therapy may be promising for the treatment of Leishmaniasis.
MATERIAL AND METHODS
Biological activity of thalidomide and liposomal amphotericin B
Leishmania used
We used two local type of L. tropica and L. donovani. Stock culture gets from Biotechnology Center / Al- Nahreen University / Baghdad. The culture is cultivated in Tobie medium17.
Parasites cultivation
1.9 ml of liquid phase was added to McCantry vials containing 5 ml of slants solid phase, then 0.1 ml of parasites inoculums were taken from stock culture through the logarithmic phase, so the initial density was 2 x 10 / ml, new promastigotes were incubated for 4 days at 26 C˚, was counting the number of promastigote by using a hemocytometer champers.
Thalidomide (Thalix-100)
Thalidomide was used as a capsule in a concentration of 100 mg made by Fresenius Kabi India get it from a local pharmacy.1000 µg/ml was prepared as a stock solution, then prepared six concentrations (10,12.5,15,20,25,30) µg/ml they were used to identify the IC50 and the IC90 for the cultured parasites.
Liposomal amphotericin B (Ambiome)
Liposomal amphotericin B also gets it from the local pharmacy as a 50mg injection concentration made by the Ireland drugs company. The stock solution was prepared as 1000 g/ml then five concentrations (5, 10, 20, 25, 30) µg/ml were used to determine IC50 and IC90. The concentrations were kept in the refrigerator at 2-8 C° 18. On the other hand, the
Synergy between the two drugs was as follow: First, the liposomal amphotericin -B was fixed at a concentration of 2.5 µg/ml, while the concentrations of thalidomide were (0.25, 0.5, 1, 2) µg/ml. Secondly, the thalidomide was fixed at a 2.5 µg/ml concentration, whereas the concentrations of liposomal amphotericin-B were (1, 2, 3, and 4) µg/ml. All concentrations were used to determine the IC50. Promastigotes numbers were identified at different time intervals (24, 48, 72, 96) hrs.
Effect of thalidomide and liposomal amphotericin -B on the morphology of L. tropica and L. donovani promastigotes
The effect of drugs on morphology was observed by using Giemsa stain for cutaneous and visceral promastigotes at IC50 concentration; then, the parasites were examined and photographed using (OPTICA, ITALY) compound light microscope supported with a camera and compared with the control group (non-infection).
Statistical Analysis
Spss version 21 software was used to analyze the data19.
RESULT AND DISCUSSION
Table (1) shows the influence of various concentrations of thalidomide on the growth of L. tropica compared with the control group through different time intervals. 10 µg/ml represents 50% of the log phase (72) hrs. Inhibitory concentration. At the same time, the highest concentration that inhibits 90% of growth was 25µg/ml.
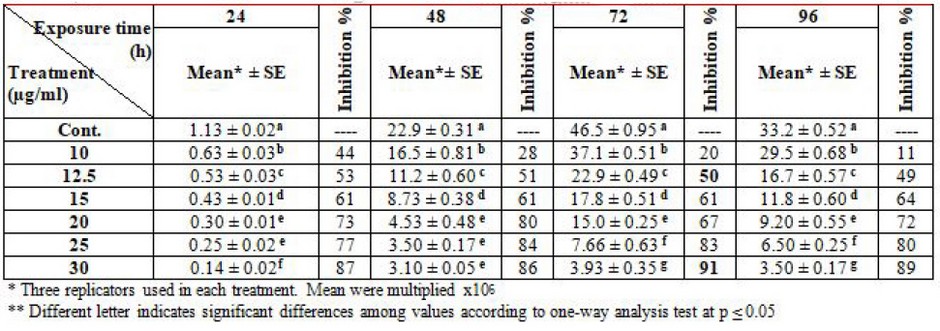
Table 1. Effect of thalidomide on the growth of L. tropica promastigotes
Table (2) display the effect of various concentrations of thalidomide on the growth of L. donovani compared to the control group through different times periods. 12.5 µg/ml appears to represent 50% the inhibitory concentration of the log phase (72) hrs. Whereas the highest concentration inhibits 90% of growth was 30 µg/ml.
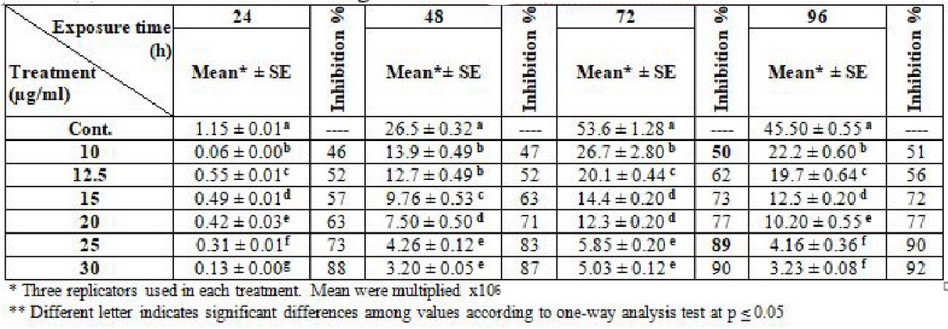
Table 2. Effect of thalidomide on the growth of L. donovani promastiotes
This study appears highly effective of thalidomide it was 25, 30 µg/ml concentrations of it to get 90% inhibitory growth and 10, 12.5 µg/ml concentrations to get 50% inhibitory growth of L. tropica and L. donovani. These results which are in agreement with20 The showed that uses of chlorpromazine as an antileishmanial agent in concentrations of 20, 25 µg/ml inhibit 90% of L. major and L. donovani growth in vitro at log
Phase. Other researchers used zinc sulfate to test the effect on L. major and L. tropica in vitro and observed that parasites in the control group were atypical growth procedures, but in the experimental group revealed a much slower growth rate 21. Another researcher indicates that synthesized pyrrolidine in different concentrations inhibits the growth of L. infantum compared with the control group22.
Thalidomide stimulates apoptotic-specific pathways via caspase 8-mediated cell death. At the mitochondrial level, they are in charge of c-junk terminal kinase (JNK) conditional release of cytochrome-c and S mac into the cytosol, where they organize the activity of the molecule that influence death apoptosis by stimulating T cells to produce IL-2, it alters natural killer cell (NK) function and numbers, thereby increasing NK-dependent cytotoxic activity 23. Thalidomide bounded to factor (TNF)-alpha inhibiting the production of interleukin (IL), a growth factor of 50% of the living cell, and they activate apoptotic pathways, causing cell death of promastigotes and decrease the growth rate to kill the promastigote cell. Thalidomide uses for cutaneous lesions treatment. Thalidomide was hypothesized to inhibit the activity of CRBN-containing E3 ligases by directly binding CRBN, 24. This study agreement with25 that the effect of some medicinal plants is used anti promastigote activity against L. aethiopica and L. donovani.
In the light of growth, the inhibitory effect of liposomal amphotericin B indicates that the lethal concentration of 50% of L. tropica was 5µg/ml, while the concentration of 20µg/ml was killed 90% of promastigotes at the log phase (72) hrs. compared with the control group, table (3)

Table 3. Effect of liposomal amphotericin -B on the growth of L. tropica promastigotes
Besides that, the inhibitory impact of liposomal amphotericin -B on L. donovani indicate the concentration of 5 µg/ml was killed at 50% of promastigotes, while the concentration of 25 µg/ml killed 90% of promastigotes at log phase (72) hrs. compared with the control group, Table (4).
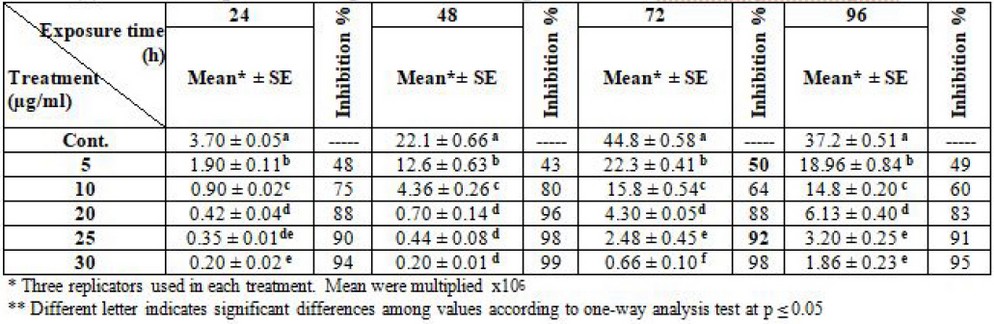
Table 4. Effect of liposomal amphotericin-B on the growth of L. donovani promastigotes
There are many studies and research about leishmaniasis treatment. Most researchers used liposomal amphotericin -B to treat L. tropica and L. donovani promastigote in India; this drug is used because it is a safe and effective therapy for visceral and cutaneous leishmaniasis 26. Additionally,27 they proved that due to the failure of existing drugs, amphotericin-B has a cure rate of 100% at a dose of 0.75-1 mg/ kg for 1-20 intravenous infusions daily. It causes severe toxicity, e.g., hypokalemia, nephrotoxicity, myocarditis, and even death, also the high cost of therapy. However, doses of 5mg/kg/day of liposomal amphotericin B caused significantly lower toxicity rates than amphotericin B lipid complex or conventional amphotericin B deoxycholate. WHO recommended that 10 or 15 mg/kg of liposomal amphotericin B have high cure rates28. This study agreement with29 reported that amphotericin-B acts by binding to ergosterol in the cell membranes; the binding might lead to modulation in cell membrane permeability, formation of pores, induction of metabolic shock, ion leakage and promotion of cell death.
Fluorescent and gold liposomes (loaded with amphotericin B or empty liposomes) were used in vitro and in vivo to elucidate the liposomes bounded with cell walls in pathogens responsible for the infection. Empty liposomes (without amphotericin) remain intact without causing cell disruption. However, the binding of amphotericin-B-loaded liposomes resulted in cell death. This may be due to the inactivation of liposomes and the secretion of amphotericin B to bind the cell membrane of ergosterol and exhibit antiparasitic activity30. Amphotericin B can translocate from the liposome to the membranes of parasite or fungal cells and bind to parasitic or fungal ergosterol higher than cholesterol29.
The inhibitory mechanism of leishmanial action is thought to be drug-binding to parasite ergosterol precursors such as lanosterol, disrupting parasite membrane, producing and changing membrane permeability, then killing parasite 27. The specialized formulation has properties that increase activity, whereas toxicity reduces: penetration and constant levels into tissues, such as the spleen and liver; high transformation temperature leading to blood, tissues and macrophages stabilization; the presence of cholesterol in the liposome, that reduces the interaction of drugs with the membranes of mammalian cells and decrease their toxicity; It is highly related to ergosterol and its precursors to ensure anti-microbial efficacy. Initial doses higher than 5mg/kg provide better penetration and more prolonged tissue continuation than repeated lower doses31.
This study shows that L. donovani required a higher dose of liposomal amphotericin-B than L. tropica promastigote. It is 20mg/kg. However, the minimum effective dose remains unknown.30. The researchers appear liposomal-loaded with amphotericin-B led to the death of cells. This possibly led to liposome disruption and amphotericin-B secretion to bind ergosterol in the cell membrane showing antiparasitic efficacy.
It is worth noting that other experiments were conducted on the effect of the two drugs together. It showed an inhibitory effect on L. tropica, when amphotericin was fixed at a concentration of 2.5 µg/ml, and thalidomide concentration changed, the IC50 was 2.5 + 0.5 µg/ml at log phase (72) hrs. Table (5).
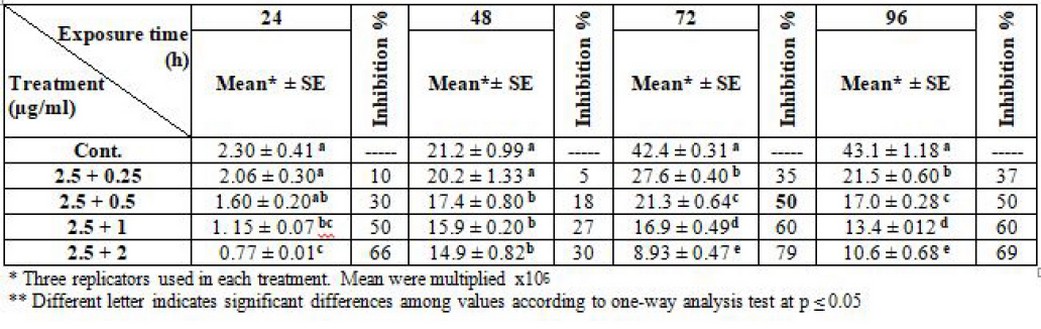
Table 5. Effect of synergism between liposomal amphotericin-B and thalidomide on L. tropica (Note: liposomal amphotericin B fixed at 2.5 µg/ml).
Additionally, the results demonstrated the inhibitory effect on L. donovani; when amphotericin was fixed at a concentration of 2.5 µg/ml, and thalidomide concentration changed, the IC50 was 2.5 + 1 µg/ml at log phase (72) hrs. Table (6).
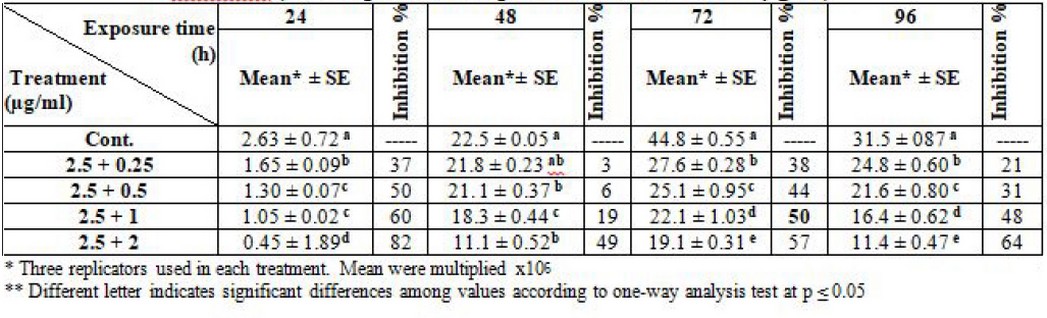
Table 6. Effect of synergism between liposomal amphotericin B and thalidomide on L. donovani (Note: liposomal amphotericin B fixed at 2.5µg/ml)
Furthermore, regarding the synergism of the two drugs, the result clarified the effectiveness of L. tropica and L. donovani promastigotes after fixation of thalidomide concentration at 2.5 µg/ml and liposomal amphotericin -B changed. The IC50 was 2.5 + 2 µg/ml at log phase (72) hrs. for the two types of parasites (table 7 and 8).
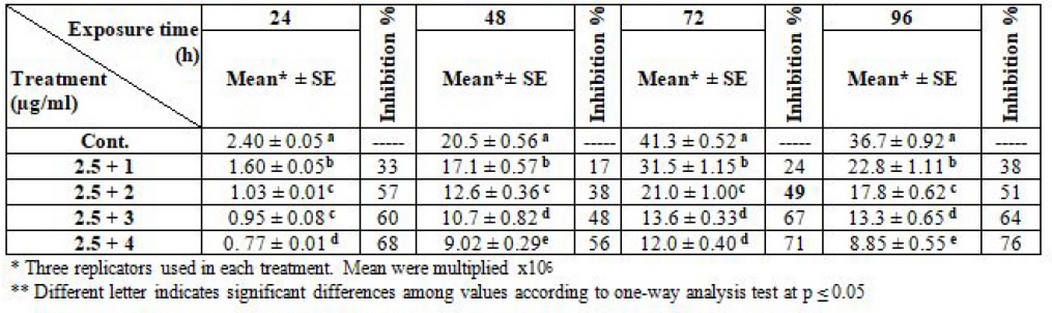
Table 7. Effect of synergism between thalidomide and liposomal amphotericin B on L. tropica (Note: thalidomide fixed at 2.5µg/ml)
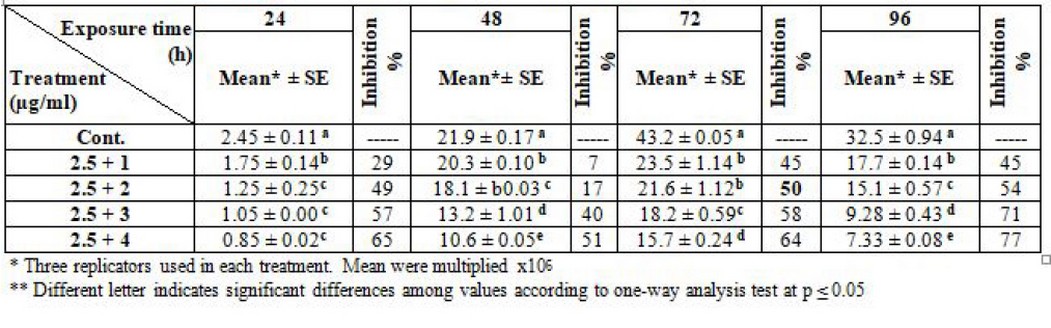
Table 8. Effect of synergism between thalidomide and liposomal amphotericin -B on L. donovani (Note: thalidomide fixed at 2.5µg/ml)
The interaction between the drugs provides a successful effect; it appears to have high toxicity on both types of leishmania promastigotes, especially when amphotericin was fixed. The synergism caused a high inhibitory effect, decay and degeneration in the living cell organelles finally, cell death and disappearance. These results are in agreement with32 they concluded that combination treatment with thalidomide and glucantim reduced significantly the disease course than single treatment with each one of the drugs in mice infected with visceral Leishmania, and this effect was dependent on the production of 1L-10 and 1FN-Y, also illustrate that thalidomide is capable of downregulating disease advancement nevertheless it didn’t completely discourage it. Others prove that the synergy between liposomal amphotericin -B and an antibacterial or antifungal agent reduces the viability of fungal cells, increases the permeability of the cell membrane, and increases cellular metabolic activity shortly after one hour of exposure33.
Thalidomide could hypothetically be included among potential drugs tested for its association with COVID-1934 based on its potent anti-inflammatory properties and activity in alleviating excessive inflammation and cytokine storms.35 confirmed the agreement to this work it was significant of the in vitro anti-leishmanial effect of the combination of allicin and AmB to reduce the required doses of the antibiotic and thus the toxicity associated with its use. Using a constant ratio analysis with the extra-cellular phase and a checkerboard analysis with amastigotes, the combination of the 2 drugs was moderate to synergistic at minimum concentrations versus both amastigotes and promastigotes; this represented an approximately two-fold decrease in the IC50 of the antibiotic added alone. The conclusion from33 appears that the synergism between liposomal AmB and other drugs causes inhibition of DNA-dependent RNA polymerase leading to the repression of RNA synthesis and cell death. This results in agreement with36 that is proved the liposomal amphotericin-B and selenium and the combination between them showed superior efficacy in the treatment of CL also noticed that no toxicity was reported.
The synergism between thalidomide and liposomal amphotericin- B appears to be more efficient in inhibiting the growth of promastigotes than when treated with each drug alone. This means that the synergism effectively inhibited both types of Leishmaniasis at lower concentrations.
It is evident from the figures (2), (3) for L. tropica and (7), (8) for L. donovani that using the treatment leads to changes in the morphological appearance from the spindle shape to the oval, circular or even irregular shape as well the occurrence of bulges and cracks in these parasites, figures (4), (5) for L. tropica and (9), (10) for L. donovani when treated with IC50 in comparing with untreated parasites, figures (2) and (6).
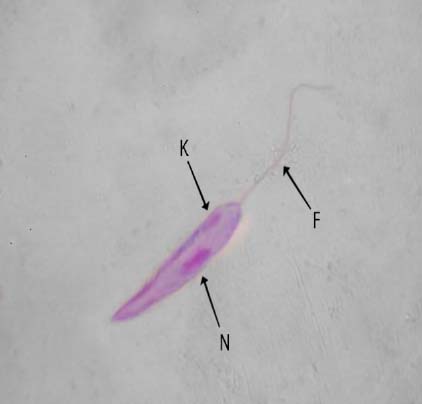
Figure 1. Untreated L. tropica N- nucleus, K-kinetoplast, F-flagellum

Figure 2. L. tropica promastigote treated IC50 of the thalidomide
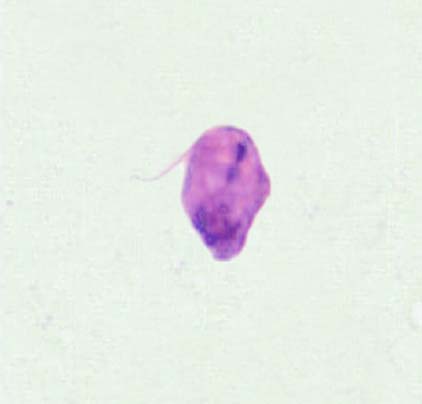
Figure 3. L. tropica promastigote treated IC50 of liposomal amphotericin-B
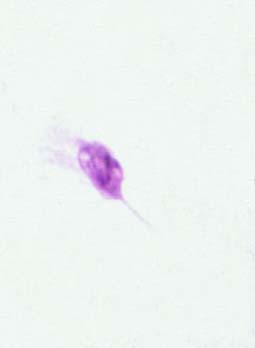
Figure 4. L. tropica promastigote treated IC50 of synergism (liposomal amphotericin-B fixed).

Figure 5. L. tropica promastigote treated IC50 of synergism (thalidomide fixed).

Figure 6. Untreated L. donovani promastigote N- nucleus, K- kintoplast, F- flagellum

Figure 7. L. donovani promastigote treated IC50 of the thalidomide note: sewellen promastigotes
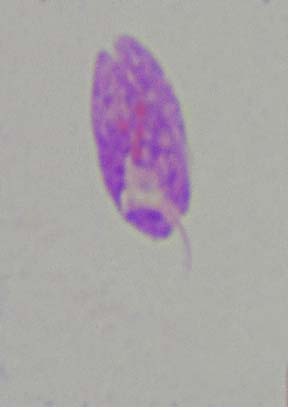
Figure 8. L. donovani promastigote treated IC50 of liposomal amphotericin -B- note, circular shape

Figure 9. L. donovani promastigote treated IC50 of synergism (liposomal amphotericin-B fixed )
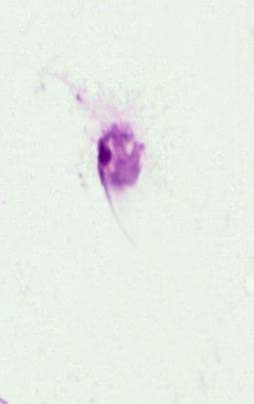
Figure 10. L donovani promastigote treated IC50 of synergism (thalidomide fixed )
The result is consistent with those manifested by37 to show that shape converted from spindle to rounded or irregular shape and became swelling; this indicates that the cell membrane permeability was affected, the osmotic cell was changed and allowed to liquid pass across the cell membrane. The inhibitory influence of the two types caused shrinkage and degeneration in the living cell organelles, finally death the cell and disappearing it in low mixed concentrations. The mechanism of liposomal amphotericin-B, considered anti Leishmaniasis, has been associated with cholesterol and distearol phosphatidyl glycerol within phospholipid bilayer synthetic and bound with protein in the medium to improve drug38. Other researchers like39 concluded the mechanism of action of clioquinol has been examined at L. infantum and L. amazonensis; results showed morphological and biochemical changes in clioquinol treated parasites, including reduction in cell size, absence of mitochondrial membrane potency, rupture of the plasma membrane, increasing the ROS production and antileishmanial activity of clioquinol versus both related species of Leishmania and suggests that the parasites’ mitochondria probably a potential biological target leading to parasite necrosis.
Change the particular enzyme or physiological activities 40; therefore, the promastigote became smallest and shortened flagellate, lost the motility and became vibrating motion then cell crash. Another research clear the effect of drugs on leishmania morphology as41 treated parasites showed significant changes in cell morphology and fluorescence emission pattern in comparison with control cells, including the appearance of abnormally round-shaped cells, lack of cellular motility, cytoplasmic enlargement and an increase in the numerical of acidophilic vesicular organelles, indicating serious physiological alterations, also showed that the cell death by apoptosis and autophagy, the parasites showing found of multi nuclei and this might affect the microtubule dynamics in species of Leishmania.
CONCLUSION
We conclude from the above that the use of drugs had high toxicity in cutaneous and visceral Leishmaniasis in vitro at low concentrations, whether for each drug alone or in the case of synergism. additionally, the treatment led to morphological changes
In these parasites, from spindle shape to the oval, circular, and irregular also, the occurrence the bulges and rupture of the plasma membrane indicate the effect on the permeability of the parasite’s cell membrane.
Funding: self-funding
Acknowledgments: The authors wish to express his gratitude to the University of Mosul, College of Science, for their provided facilities, which helped to improve the quality of this work.
Conflicts of Interest: Authors declare that they have no conflict of interest for this study.
REFERENCE
1. Alemayehu, B. and Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. J. Heal. sci.,2017; 11(4): 519.
2. Torres-Guerrero, E.; Quintanilla-Cedillo, M. R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review Version 1; Peer review: 2 approved. J. F1000Res., 2017; 6: 750. doi: 10.12688/f1000research.11120.1.
3. Chagas, E. C. S.; Silva, A. S.; Ferreira, N.; Ferreira, L. S.; Sampaio, V. S.;Terrazas, W. C. M.; Guerra, J. A. O.; Souza, R. A. F.; Silveira, H. and Guerra, M. G. B. Compostion of Sand Fly Fauna (Diptera:Psychodidae) and Detection of Leishmania DNA(Kinetoplastida :Trypanosomatidae) in Different Ecotopes from Arural Settlement in the Central Amazon, Brazil. J. Parasit. Vect. 2018; 11:18. https://dio.org/10.1186/s13071-018-2743-6
4. Mann, S.; Frasca, K. Scherrer, S.; Henao-Martinez, A. F.; Newan, S.; Ramanan, P. and Suarez, J. A Review of Leishmaniasis:Current Knowledge and Future Directions. J. Cur. Tro. Med. Rep.,2021; 8:121-132.
5. Choi, H. L.; Jain, S. and Jose, A.; Postigo, R.; Borsch, B.; Dagne, D. A. The Global Procurement Landscape of Leishmaniasis. J. plos. Negl. Trop. Dis.,2021; 15(2): e0009181. https://dio;10.1371/journal.
6. Nampoothiri, R. V.; Sreedharanunni, S.; Chhabria, BA and Jain,S. Visceral Leishmaniasis: Kala-azar. An. Inter. J. med., 2016;109(5): 347-348.
7. William, H.; Markle, M. D. and Makhoul,K, M. D. Cutaneous Leishmaniasis: Recognition and Treatment. J. Am. Fam. Physician.2014; 69(6): 1455-1460.
8. Abadias-Granado, A.; Diago, P.A.; Cerro, A.M.; Palma-Ruiz, Y.; Gilaberte, A. Cutaneous and Mucocutaneous Leishmaniasis. J. AcTAS. Dermo-Sifiliograficas.2021; 112(7): 601-618.
9. Uliana, SRB; Trinconi, C. T. and Coelho, A. Chemotherapy of Leishmaniasis: present Challenges. J. Parasitol.,2017;145(4)., Doi: https://doi.org/10.1017/s003//820/62523.
10. Tiuman, T. S.; Santos, A. O.; Ueda-Nakamura, T.; Filho, B.D.; Nakamura, C. V. Recent Advances in Leishmaniasis Treatment. J. Infect. Dis. 2011; 15(8): 525– 532.
11. de Menezes, J. P. B.; Guedes, C. E. S.; Almeida Petersen A. L. D. O.; Fraga, D. B. M. and Veras, P. S. T. Advances in Development of New Treatment for Leishmaniasis. J. Biomed. Res. Int.,2018; http://dx.doi.org/10.1155/2015/815023.
12. Croft, S. L. and Coombs, G. H. Leishmaniasis – Current Chemotherapy and Recent Advances in the Search for Novel Drugs. J. parasitol.,2003; 19(11): 502-508.
13. Ghorbani, M. and Farhoudi, R. Leishmaniasis in humans: drug or vaccine therapy. J. Dove. Press.,2018; 12: 25-40.
14. Ghobrial, I. M. and Rajkumar, S. V. Mangment of Thalidomde Toxicity. J. Supo. Onco.,2003; 1(3): 194-205.
15. Upputuri, B.; Pallapati, M. S. Tarwater, P and Srikantam, A. Thalidomide in the Treatment of Erythema Nodosum Leprosum (ENL) in Anoutpatient Setting. J. PLoS. Negl. Trop. Dis.,2020;14(10): e0008678. Dio:10.1371 https://doi.org/10.1371/.
16. Moen, M.; Lyseng-Williamson, K. A. and Scott, L. J. Liposomal Amphotericin B: a Review of its use as Empirical Therapy in Febrile Neutropenia and in the Treatment of Invasive Fungal Infections. J. Adis. Dru. Eval.,2012; 69: 316-392.
17. Tobie, E. J.; Brand, T. V. and Mehlman, B. Cultural and Physiological Observations on Trypanosoma rhodesiense and Trypanosoma gambiense. J. Parasitol.,1950; 36(1): 48-54.
18. Singodia, D.; Verma, A.; Khare, P.; Dube, A.; Mitra, K. and Mishra, P. Rl. Investigations on Feasibility of in Situ Development of Amphotericin B Liposomal for Industrial Applications. J. lipo Res.,2012; 22(1): 8-17.
19. Moore, E. M. and Lockwood, D. N. Treatment of Visceral Leishmaniasis. J. Glo. Infec. Dis.,2010; 2(2): 151-158.
20. Al-Healy, H. L. and Al-Chalabi, K. A. Effect of Chloropromazine Hydrochloride on L. major and L. donovani Content of Nucleic Acids and Proteins. J. Raf. Sci.2002; 13(1): 137-146.
21. Bafghi, A. F.; Noorbala, M.; Noorbala, M. T. and Aghabagheri, M. Antileishmanial Effect of Zinc Sulphate on the Viability of Leishmania tropica and leishmania major Promastigotes. J. Micro-biol.,2014; 7(9) e11192. DOI:105812/jjm.11192.
22. Al-Hayali, H.; Al-Hamadany,A. J. and Al-Kattan, M. M. pEffect of Synthesized Pyrrolidines in Growth of Leishmania infantum Promastigotes. J. T. P. Sci., 2018; 23(7): 55-62.
23. Anderson, C. Lenalidomide and Thalidomide: Mechanisms of Action-Similarities and Differences. J. Semin. hematol., 2005;42: 53-58.
24. Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y and Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. J. sci.,2010; 327(5971): 1345.
25. Nigatu, H.; Belay, A.; Ayalew, H.; Abebe, B.; Tadeese, A.; Tewabe, y. and Degu, A. In Vitro Antileishmanial Activity of Some Medicinal Plants. JDOPR.2021; 13: 15-22.
26. Wijnant, G. J.; Bocxlaer, K. V.; Yardley, V.; Harris, A.; Alavijeh, M.; Pedosa, R. S.; Antuns, s.; Mauricio, I.; Murdan, s. and Croft S. L. Comparative Efficacy, Toxicity and Biodistribution of the Liposomal Amphotericin B Formulations Fungisome and AmBisome in Murine Cutaneous Leishmaniasis. J. I. Parasitol. Drus. Dru. Res.,2018; 8(2): 223-228.
27. Sundar, S and Chakravarty, J. Lipsomal Amphotericin B and Leishmamiasis: Dose and Reponse. J. Gl. Inf. Dis.,2010; 2(2). DOI:10.4103/ 0974 -777X.62886.
28. WHO. Report of a WHO Information on sultation on Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis. 2005;16 April, Roma, Italy.
29. Shirzadi, M. R. Liposomal Amphotericin B: A Review of its Properties, Function, and use for Treatment of Cutaneous Leishmaniasis. J. Do. Pr., 2019;10: 11-18.
30. Stone, N. RH.; Bicanic, T.; Salim, R. and Hope, W. Lipoomal Amphotericin B (AMBisome): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. J. Dru., 2016;76: 485-500.
31. Saravolatz, L.; Bern, C.; Adler-Moore, J.; Berenguer, j.; Boelaert, M.; Denboer, M.; Davidson, R. N.; Figueras, C.; Gradoni, L. and Kafetzis, D. A.. Liposomal Amphotericin-B- for the Treatment of Visceral Leishmaniasis. J. Clin. Info. Dise. 2006;43(7): 917-924.
32. Solgi, G.; Kariminia,A.; Abdi, K.; Darabi, M. and Charechozloo, B. Effects of Combined Therapy with Thalidomide and Glucantime. on Leishmaniasis Induced by L. major in BALB/c mice J. Par. Logy.,2006; 44(1): 55-61.
33. Teixeira-Santos, R.; Ricardo, E.; Branco, R. J.; Azevedo, M. M.; Rodrigues, A. G. and Pina-Vaz, C. Unveiling the Synergistic Interaction Between Liposomal Amphotericin B and Colistin. J. Fro. Micr. Biol., 2016; 7(1439): doi. 10.3389 /fmicb. 2016. 01439.
34. Khalil, A. Kamar, A. and Nemer, g. Talidomide-revisited: are COVID-19 Patients Going to be the Latest Victims of yet Another Theoretical Drug- Repurposing. J. Fr.Imm doi.org /10.3389/ fimmu 2020.01248
35. Corral, M. J.; Gonzalez-Sanchez, E.; Cuquerella, M. and Alunda, M. In Vitro Synergistic Effect of Amphotericin B and Allicin on L. donovani and L. infantum. J. Asm. Org.,2014; 58(3): 1596-1602.
36. Mostafavi, M.; Farajzadeh, S.; Sharifi, I.; Khazaeli, P.and Sharifi, H. Leihmanicidal Effects of Amphotericin B in Ombination with Selenium Loaded on Noisome Againt Leishmania tropica. J. P. D.,2019; 43(2): 176-185.
37. Al- Khan, H. I.; Al-Hayaly, H. L and Al- Mushhadani, T. M. Effect of Aqueous Extract of Nerium oleander and Melia azedarach plants on the Morphology and Osmolarity of Leishmania tropica promastigotes n Vitro. «Rafi. J. of Sci»2006; .17(13): 68-79.
38. Yardley, V.; Croft, S. L. Actvity of Liposomal Amphotericin -B Against Expermintal Cutaneous Leishmaniasis. J. Ant. Age. Chem.1997; 41(4):752-756. https://doi.org/10.1128/AAC.41.4.752.
39. Tavares, G. S..; Mendonca, D. V. C.; Lage, D. P.; Granato, J. T.; Ottoni F. M.; Ludolf, F.; Charez-Fumagalli, M. A. C.; Duarte,M. C.; Taraves, C. A. P.; Alves, R. J.; Coimbra, E. S. and Coelno, E. A. F. Anti Leishmanial Activity, Cytotoxicty and Mechanism of Action of Clioquinol Against Leishmania infantum and Leishmania amazonensis Species. J. Org., 2018;123(3):236-246.
40. Al-Hayali, H. L.; Al-Hammoshi, M. H. and Al-Mushhadani, T. M. Synthesis and Study Effect of 3,4-Dihydro-4-(p-anis)-6-Phenyl Pyrimidine 2(1H)-One on Growth and Morphology of Leishminia tropica Promastigote in Vitro. Tikrit J. Pure Sci., 2008;13(3): 209-218.
41. Neto, R. L. M.; Sousa, L. M. A.; Dias, C. S.; Filho, J. M. B.; Oliveira, M. R. and Figueiredo, R. C. B. Q. . Morphological and physiological changes in Leishmania promastigotes:215 induced by yangambin, a lignan obtained from Ocotea duckei. J. Exper.Pars.,2011; 127: 215 -221
Received: 8 February 2022 / Accepted: 12 April 2022 / Published:15 May 2022
Citation. Al-Mamary NI , Al-Hayali HL. Effect of Synergism of Thalidomide and Liposomal Amphotericin-B on Leishmania tropica and Leishmania donovani Promastigote. Revis Bionatura 2022;7(2) 59. http://dx.doi.org/10.21931/RB/2022.07.02.59



















