Vol 7 No 2 2022- 53

1,2College of pharmacy / The University of Mashreq, Baghdad-Iraq
3College of Pharmacy/ Tikrit University, Tikrit-Iraq
*Corresponding author e-mail: phphloma@gmail.com,
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.53
ABSTRACT
The coronavirus (SARS-CoV-2) that causes Coronavirus disease 19 (COVID-19) has recently emerged as a cause of severe infection in a considerable percentage of infected persons. Predicting the risk factors for severe disease can greatly help manage critical cases and save lives. This study aimed to assess the prognostic value of the platelet-lymphocyte ratio (PLR) and C-reactive protein (CRP) in patients with COVID-19. This cross-sectional study enrolled 160 confirmed cases with COVID-19 by real-time polymerase chain reaction. Demographic data (age, gender, smoking status, body mass index (BMI)) and comorbidity were collected through direct interviews. Laboratory investigations, including total leukocyte count, absolute neutrophil, lymphocyte, platelet count, serum level of C-reactive protein, and hemoglobin, were obtained from the patient’s records. The platelets-lymphocyte ratio was calculated by dividing absolute platelet count by absolute lymphocyte count. According to their outcome, patients were categorized into two groups: those discharged well and those who required intensive care unit (ICU) admission. Out of 160 included patients, 32 (20%) needed ICU admission due to the deterioration of their status. Age (64.28±13.08 years versus 57.43±13.15 years), hypertension (40.63% versus 20.31%) absolute neutrophil count (median = 12.9×103/ml, range 3.83-22.8×103/ml versus median=6×103/ml, range 2.17-22.8×103/ml) and PLR ((median= 257.27, range= 62.72-1072 versus median= 191.54, range= 17.85-919.12) were significantly higher in patients required ICU admission than those discharged well, and associated significantly with the severity of the disease. Advanced age, hypertension, neutrophilia, and PLR at admission are predictors of severity and need for ICU admission in patients with COVID-19. PLR is an inexpensive, easy-to-be-calculated parameter that can be used routinely to predict the severity of COVID-19.
Keywords. COVID-19, intensive care unit, platelet-lymphocyte ratio
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 with a wide range of symptoms, from asymptomatic to severe pneumonia and acute respiratory distress syndrome (ARDS) or even severe symptoms of death 1. Immunological investigations have demonstrated that high concentrations of proinflammatory cytokines (cytokine storm) are the hallmark characteristic of severe COVID-19 cases. This unusual rise of cytokines associated with a tremendous proinflammatory response leading to Multiple Organ Dysfunction Syndrome (MODS) and ARDS, which are the leading causes of death in patients with COVID-19 2. Thus, theoretically, inflammatory markers could be used to measure the severity and death risk of COVID-19 patients. Platelet-to-lymphocyte ratio (PLR) is a new marker of inflammation, which is cheap and easily obtained from resource-limited settings. This ratio has been used to diagnose various diseases, such as autoimmune diseases and heart diseases 3. Previous studies disclose that severe COVID-19 patients had a rise in PLR at admission 4. This proposes the potential prognostic value of this ratio in COVID-19 patients, especially in poor settings. C-reactive protein is a protein formed by the liver 5. Usually, the serum level of CRP is less than 10 mg/L; however, it rises rapidly when there is an inflammatory process. Its half‐life is about 19 hours 6, and its concentration drops when the inflammatory stages end. CRP preferably links to phosphocholine, which is expressed on the surface of injured cells 7. This linking activates the classical complement pathway of the immune system. Therefore, CRP could be a valuable marker for monitoring COVID-19 severity 8. The present study aimed to assess the prognostic value of PLR and CRP in patients with COVID-19.
MATERIALS AND METHODS
Patients and Methods
This was a prospective cross-sectional study including 212 patients suspected of having an infection with SARS-CoV-2 who were admitted and treated at Al-Shifaa Hospital/ Baghdad from 1st May to 1st October 2020. Patients were diagnosed after nasopharyngeal swab examination of SARS-CoV-2 RNA by real-time polymerase chain reaction (RT-PCR). Patients with asthma or COPD, and those with known coagulopathies and autoimmune diseases, were excluded from the study. Thus, according to RT-PCR and exclusion criteria, the eligible patients were 160 patients who represented the study population. The review board approved this study at the University of Mashreq. Written consent from each participant was obtained before sample collection after explaining the aim of the study. Each patient was given the complete unconditioned choice to withdraw anytime. The confidentiality of data throughout the study was guaranteed, and the patients were assured that data would be used for research purposes only. Socio-demographic and clinical data, including age, sex, body mass index (BMI), and smoking, were collected through direct interviews.
Sample Collection and Laboratory Investigations
Three mL of peripheral blood were collected from each participant. A hematology auto-analyzer (Huroba ABX/India) measured blood parameters. PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte.
Follow-up
Patients were followed up for one month after hospital admission. The need for ICU admission or death was reported. Accordingly, patients were categorized into two groups: those who were discharged well and those who required ICU admission or died.
Statistical analysis
All statistical analyses were performed using SPSS statistical software, version 25 (SPSS, IBM Company, Chicago, USA). The normal distribution of continuous data was tested with the Shapiro Wilk test. Normally distributed variables were presented as mean ± SD and analyzed with the Student t-test. Non-normally distributed variables were presented as median and range and analyzed with a nonparametric Mann Whitney U test. Categorical variables were expressed as counts and percentages and analyzed with the Chi-square test. Receiver operating characteristic (ROC) curve analyses were used to evaluate the prognostic value of PLR. A significant level of statistics was considered for all tests when p<0.05.
RESULTS
Demographic Characteristics of the Patients
The mean age of the patients was 61.05±12.57 years; among them were 96 males (60%). Smoking habit was encountered in 13.75% of the patients. The mean BMI was 25.34±3.85 kg/m2. Hypertension was the most common comorbidity affecting about a quarter of the patients (24.38%), followed by T2DM (22.5%). The mean total WBC, neutrophil, lymphocyte and platelet count was 14.1±5.06×103/ml, 11.34±4.82×103/ml, 1.33±0.79×103/ml and 261.5±111.17×103/ml, respectively. Hemoglobin concentration ranged from 7.8-17.0 g/dl with a mean of 12.18 ±1.5 g/dl. The mean PLR and CRP were 297.16±215.3 and 50.11±36.56 ng/ml, respectively (Table 1).

BMI: body mass index, WBC: white blood cells, T2DM: type 2 diabetes mellitus, IHD: ischemic heart disease, PLR: platelet- lymphocyte ratio, Hb: hemoglobin, CRP: C-reactive protein
Table 1. Patients’ characteristics and demographic data (n=160)
Outcomes
Out of 160 included patients, 32 patients (20%) required ICU admission and 8 patients (5%) of those died during the follow-up period. The other 128 patients (80%) were discharged well.
Association of Demographic Factors with the Outcome
Four demographic and clinical factors were significantly associated with ICU admission in patients with COVID-19. The mean age of the ICU group was 64.28±13.08 years which was higher than those discharged well (57.43±13.15 years), with a highly significant difference. The frequency of hypertension in the ICU group and the well-discharged group was 40.63% and 20.31%, respectively, with a significant difference. The median neutrophil count in the ICU group was 12.9×103/ml (range 3.83-22.8×103/ml) compared with 11.6×103/ml (range 2.17-22.8×103/ml) in the well-discharged group with a significant difference. Finally, ICU group had significantly higher PLR than well-discharged group (median= 257.27, range= 62.72-1072 versus median= 191.54, range= 17.85-919.12) as shown in table 2
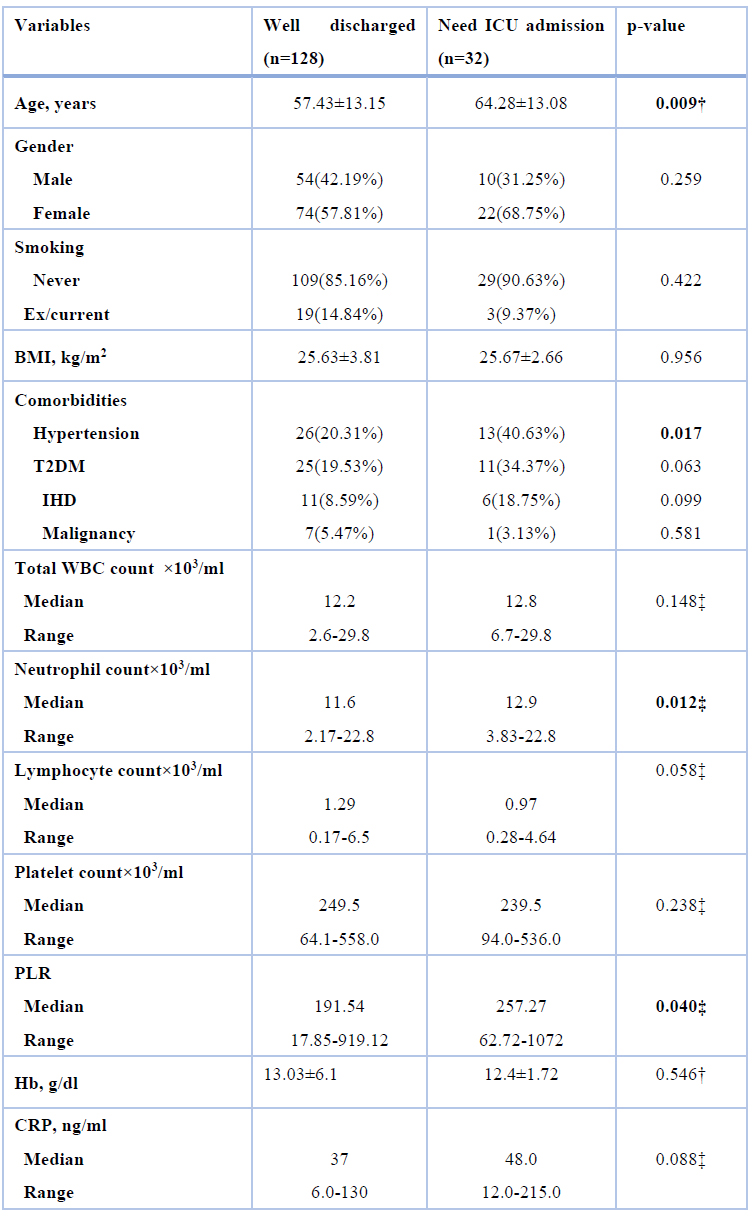
BMI: body mass index, WBC: white blood cells, T2DM: type 2 diabetes mellitus, IHD: ischemic heart disease, PLR: platelet- lymphocyte ratio, Hb: hemoglobin, CRP: C-reactive protein
† Student t-test, ‡ Mann Whitney test, Values were expressed as mean±SD unless indicated otherwise
Table 2. Association of demographic factors with the outcome
Prognostic Value of PLR
The receiver operating characteristic (ROC) curve was used to evaluate the prognostic value of the platelet-lymphocyte ratio in COVID-19. The area under the curve (AUC) was 0.617, 95%CI= 0.510-0.725, p= 0.040. The sensitivity and specificity of the test at cut-off values of PLR= 235.28 were 0.63 and 0.60, respectively (Figure 1).
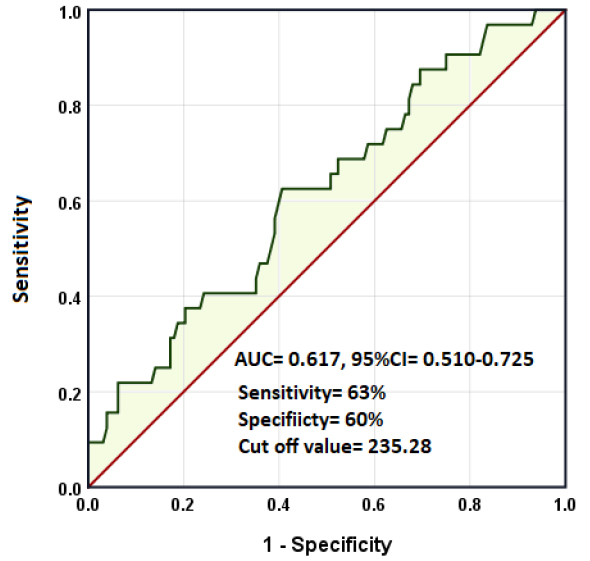
Figure 1. Receiver operating characteristic curve for PLR in predicting worse outcomes in patients with COVID-19
DISCUSSION
The present study aimed to assess the prognostic value of CRP and PLR in patients with COVID-19. According to the result of the study, 20% of patients needed ICU admission, among whom 5 patients (25%) died. These figures are slightly lower than in the global context. In a large meta-analysis including 646 studies with 24983 patients, Abate et al.9 demonstrated that 32% of the patients required ICU admission, and the mortality rate among those was 39%. The variation may explain this discrepancy in the demographic and clinical features of the patients, especially age and the presence of comorbidities.
In the present study, advanced age, hypertension, leukocytosis, ad admission were predictors for worse outcomes in COD-19 patients. Advanced age may be the most risk factor confirmed as a predictor of mortality in different studies worldwide. In a large Spanish cohort involving 2226 patients, the worse outcome for younger patients was 0.5 % for those below 40 years, 1.5 % for those 40–49 years, and 3.8 % for those 50–59 years 10. In another study, Lei et al.11 investigate the outcome of 288 Chinese patients in a retrospective study. The authors found that each 1-year increase in age increases the chance of ICU admission by 1.07-time. Although the pathophysiological mechanisms are still not understood, they may be explained by the dysfunction of the immune system with aging 12. In addition, a study reported that older age was related to defects in T-cell and B-cell function and excess inflammation markers, which could be detrimental to the control of viremia and inflammation, aggravating morbidity and mortality in older patients 13. Also, in line with the present study about the role of hypertension are many studies worldwide. Wang et al.14 reported 138 COVID-19 patients hospitalized in Wuhan, China: 31% had hypertension. Of those requiring ICU admission, 58% had hypertension compared with 22% who did not. Guan et al.15 reported 1099 COVID-19 cases across China: 15% of all patients had hypertension, including 24% in severe cases and 13% in mild cases. Among patients admitted to an intensive care unit (ICU) who required tracheal intubation or died, 36% had hypertension. This association between hypertension and disease severity may be explained based on two facts: firstly, hypertension is associated with more severe COVI-19. Supporting this fact are many studies worldwide. In a cohort of 1389 patients, a history of hypertension was more common among those who had severe than non-severe COVID-19 16. Similarly, in a separate cohort of 1590 hospitalized patients in China, underlying hypertension was independently associated with severe COVID-19 17.
There is no precise mechanism(s) that explains this association between hypertension and the severity of COVID-19. However, antihypertensive medications based on Renin-Angiotensin-Aldosterone System (RAAS) inhibition, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), that use in the treatment of hypertension can upregulate the expression of ACE2 in the kidney tubules 18. Therefore, they could hypothetically increase the virus’s targeting of some vital organs like kidneys. This explains why hypertension but not diabetes was significantly associated with disease severity in those patients. In most studies, neutrophilia was a common finding in severe patients. A Singaporean study including 138 hospitalized patients showed that neutrophilia was significantly higher in patients requiring admission to ICU (11.6×103/ml vs. 3.5×103/ml) 19.
Similarly, an Ethiopian study showed that neutrophilia was more prominent in severe and critical than moderate patients 20. Qin et al.12 and Gong et al.21 reported significantly higher neutrophil count in severe than non-severe patients. Another study by Zhang et al.22 revealed that 74.3% of dead patients had neutrophilia on admission, increasing to 100% 24 hours before death. The presence of neutrophilia could be related to the cytokine storm that characterizes COVID-19 disease. However, careful interpretation is required as neutrophilia could be due to bacterial co-infections 20. Another important finding in the present study was that high PLR at admission was significantly associated with ICU admission despite that platelet count was higher in patients who did not need ICU admission. Following this result is a meta-analysis of seven studies with 998 participants. The study showed that severe COVID-19 patients had higher PLR levels on admission 23. Yang et al.24 reported the optimal cut-off PLR value as 180 with an AUC of 0.784, specificity of 44%, and sensitivity of 77%.
Meanwhile, Sun et al.25 suggested a cut-off PLR value of 226.67 with an AUC of 0.746, specificity of 80.90%, and sensitivity of 59.26%. This discrepancy warrants further research to determine the most appropriate PLR cut-off value in determining the severity of COVID-19 patients. PLR was initially suggested as an excellent candidate marker for determining the severity and mortality of COVID-19. First, PLR is an established marker of inflammation 26.
Inflammation plays a considerable role in the pathophysiology of COVID-19, with cytokine storm as a hallmark condition in severe disease and poorer prognosis 27. Thus, a high PLR value suggests an overactive inflammatory response and a worse prognosis. Second, PLR is sensitive to natural and acquired immune responses because it includes the absolute number of lymphocytes, the leading player in immune response 28. Third, PLR is an inexpensive and readily available measurement used in resource-limited settings. The underlying mechanism for the decreased absolute lymphocyte count is thought to be due to induction by SARS-CoV-2 of pyroptosis in lymphocytes through the activation of nod-like receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome 24. The present study showed no significant association of CRP with the severity of COVID-19. This result agrees with a Serbian study involving 128 patients, in which there was no significant difference in CRP concentration between patients with different severity of the disease 29. However, many other studies do find such differences. For example, elevated CRP values have been reported in viral respiratory illnesses such as SARS, MERS-CoV, and H1N1 and have been reported to correlate with disease severity and predictors of disease progression 30,31. Similarly, CRP levels have been elevated in hospitalized patients with COVID-19 and correlate with the severity of the disease and mortality 32. This discrepancy between different studies is mainly attributed to the underlying comorbidities that may enhance CRP production.
CONCLUSION
these data suggest that advanced age, hypertension as a comorbidity, higher neutrophil absolute count, and PLR ad admission are predictors of severity and need for ICU admission in patients with COVID-19. PLR is an inexpressive, easy-to-be-calculated parameter that can be used routinely to predict the severity of COVID-19. However, a more reliable conclusion requires further studies with larger sample size.
Acknowledgments
The author is grateful to all staff members of Al-Shifaa Hospital for their help and cooperation
Conflict of Interest
The author declares that she has no competing interests.
Fundus: Self
REFERENCES
1. Huang, C.; Wang, Y.; Li, X, Ren L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020,395(10223):497–506.
2. Yuki K, Fujiogi M, Koutsogiannaki S. (2020). COVID-19 pathophysiology: a review. Clin Immunol. 215:108427.
3. Gasparyan, AY.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med 2019, 39: 345–357.
4. Yang, A.P.; Liu, J.P.; Tao, WQ.; Li, H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020,84: 106504.
5. Marnell, L.; Mold, C.; Du Clos, T.W . C‐reactive protein: ligands, receptors, and role in inflammation. Clin Immunol. 2005,117(2):104‐111.
6. Pepys MB, Hirschfield GM. (2003). C‐reactive protein: a critical update. J Clin Invest. 111(12):1805‐1812.
7. Sproston, N.R.; Ashworth, J.J. . Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018, 9:754.
8. Young, B.; Gleeson. M.; Cripps, A.W. C‐reactive protein: a critical review. Pathology 1991,23(2):118‐124.
9. Abate, S.M.; Ahmed Ali, S.; Mantfardo. B.; Basu, B. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One 2020,15(7):e0235653.
10. Borobia, A.M.; Carcas, A.J.; Arnalich, F.; Álvarez-Sala, R.; Monserrat-Villatoro, J.; Quintana, M. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020,9:1733.
11. Lei, M.; Lin, K.; Pi, Y.; Huang, X.; Fan, L.; Huang, L. Clinical features and risk factors of ICU admission for COVID-19 patients with diabetes. J Diabetes Res 2020:ID 5237840.
12. Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infec Dis. 2020,71(15):762–768.
13. Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Zhibo, L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. .Lancet 2020,395(10229):1054–1062.
14. Wang, Y.; Shang, J.; Graham, R.; Baric, RS.; Li, F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 2020,94(7):e00127-20
15. Guan, W.J.; Liang, W.H.; Zhao. Y.; Liang, HR.; Chen, ZS.; Li, Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020,55: 2000547
16. Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020,52:1193.
17. Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, C.; Ou, J.; He, L. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020
18. Angel-Korman, A.; Brosh, T.; Glick, K.; Leiba, A. COVID-19, the kidney and hypertension. Harefuah. 2020,159(4):231-234.
19. Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020,95:131–134.23.
20. Araya, S.; Wordofa, M.; Mamo, M.A.; Tsegay, Y.G.; Hordofa, A.; Negesso, A.E. The Magnitude of Hematological Abnormalities Among COVID-19 Patients in Addis Ababa, Ethiopia. J Multidiscip Healthc. 2021,14:545-554.
21. Gong, J.; Ou, J.; Qiu, X.; Jie, Y.; Yuan, L.; Cao, J. A tool to early predict severe 2019-novel coronavirus pneumonia (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020,71(15):833-840.31.
22. Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J. Clinical characteristics of 82 death cases with COVID-19. PLoS ONE. 2020,15(7):e0235458
23. Simadibrata, D.M.; Pandhita, B.A.; Tango, EM. Platelet-to-lymphocyte ratio, a novel biomarker to predict the severity of COVID-19 patients: a systematic review and meta-analysis. J Intensive Care Society 2020,0(0):1–7
24. Yang, M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. 2020. http://dx.doi.org/10.2139/ssrn.3527420
25. Sun, S.; Cai, X.; Wang, H.; He, G.; Lin, Y.; Lu, B. . Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta 2020,507: 174–180
26. Akboga, M.K.; Canpolat, U.; Yuksel, M.; Yayla, C.; Yilmaz, S.; Turak, O. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: a single center large-scale study. Platelets 2016,27: 178–183.
27. Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020,20: 355–362.
28. Kartal, O.;Kartal, A.T. . Value of neutrophil to lymphocyte and platelet to lymphocyte ratios in pneumonia. Bratisl Lek Listy 2017,118: 513–516.
29. Cekerevac V, Turnic TN, Draginic N, Andjic M, Zivkovic V, Simovic S. (2021). Predicting severity and intrahospital mortality in COVID-19: the place and role of oxidative stress. Oxidative Med Cellular Longevity 2021 |Article ID 6615787
30. Ko, J.H.; Park, G.E.; Lee, J.Y.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016,73(5):468–75.
31. Vasileva, D.; Badawi, A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm Res 2019,68(1):39–46.
32. Sharifpour, M.; Rangaraju, S.; Liu, M.; Alabyad, D.; Nahab, F.B.; Creel-Bulos, C.M. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS ONE 2020,15(11): e0242400.
Received: 20 February 2022 / Accepted: 9 March 2022 / Published:15 May 2022
Citation. Mahmood Edan L, Samein L H,. Salih K S.. Prognostic Value of C-Reactive Protein and Platelet Lymphocyte Ratio in Coronavirus Disease 19.Revis Bionatura 2022;7(2) 53. http://dx.doi.org/10.21931/RB/2022.07.02.53




















