Vol 7 No 2 2022- 48

1 Department of Dentistry, Dijlah University College, Baghdad, Iraq.
2Department of Dentistry, Dijlah University College, Baghdad, Iraq.
3Material Research Directorate, Ministry of Science and Technology, Baghdad, Iraq.
4Department of Dentistry, Dijlah University College, Baghdad, Iraq.
*Corresponding Author: Ghufran D. Awad e-mails: ghufran.dhari@duc.edu.iq,
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.48
ABSTRACT
Orthodontic treatment improves dental aesthetics. However, the prolonged duration of treatment and patient’s oral hygiene results in more significant biofilm buildup and boosting concentrations of acidogenic bacteria, resulting in enamel demineralization. Plasma treatment technology has developed an interest in enhancing appliances’ surface properties. The present study aimed to investigate cytotoxicity and antibacterial activity represented by anti-adhesion and anti-biofilm formation of Argon plasma treatment on orthodontic brackets against Streptococcus mutans and Lactobacillus acidophilus and evaluation the effect of change in plasma exposure duration on these properties in vitro. The study included three groups, group 1: untreated stainless steel brackets, group 2: brackets treated for 15 min, and group 3: brackets treated for 30 min. S.mutans and L.acidophilus were isolated from patients undergoing fixed orthodontic treatment. Microbiological tests included adhesion assay using plate counting method and biofilm formation assay using ELISA plate reader. S. mutans& L. acidophilus were cultivated in three groups, and their adherence and biofilm formation were measured and compared. The biocompatibility of treated stainless steel brackets was evaluated by MTT assay. Results showed that groups 2 & 3 had better anti-adhesion and anti-biofilm activity when compared with group 1, with a significant difference between group 1 &group 3, in which group 3 recorded the lowest biofilm formation and the highest anti- anti-A adhesion activity. MTT showed that groups 2 and 3 exhibited more than 80% cell viability after 24, 48, and 72 hours. Plasma surface treatment of metal brackets possesses antibacterial activity against S.mutans and L.acidophilus; alteration exposure time impacts the development of these properties.
Keywords. Plasma treatment, Argon gas, orthodontic brackets, biofilm, antimicrobial.
INTRODUCTION
Recently, the demand for orthodontic treatment has risen due to increased population awareness about the role of orthodontic treatment in improving dental and facial aesthetics and, consequently, their popularity and social acceptance 1. Fixed orthodontic appliance parts like brackets, bands and archwires make tooth brushing difficult and create ideal circumstances for the formation of dental biofilm and the colonization of bacteria. The biofilm formation can cause periodontal problems and enamel decalcification, which affect nearly half of all orthodontic patients 2. In orthodontic-induced enamel decalcification, the initial bacterial adherence to the bracket-adhesive-enamel interface is crucial. The continued growth of these initially adhering bacteria that were only partially removed by tooth brushing might contribute to the establishment of a pathological oral biofilm. The organic acids generated by adhering bacteria such as S.thoraltensis, S.epidermidis, Bacillus spp., Neisseria spp., S. aureus and M. luteus when they come in contact with the tooth surface and left for enough time can cause demineralization of the enamel and the formation of white spot lesions (WSL), which are usually apparent clinically in the first month after orthodontic appliances are placed 3. Streptococcus mutans and Lactobacillus acidophilius have significant components of the pathogenic bacterial flora in WSLs and dental caries 4. While S. mutans has been linked to the onset of dental caries, studies suggest that lactobacilli may have a more crucial role in the progression of caries 5. The surface free energy and surface roughness of the implant surfaces play a crucial role in bacterial adherence and colonization. In research conducted by Eliades and h 6, they found that stainless steel has greater critical surface tension and energy, which can be thought to have a more excellent plaque retaining ability.
Furthermore, metallic orthodontic brackets altered the oral environment, such as pH level reduction and increased colonization of Streptococcus mutans and Lactobacillus acidophilus 7. Various methods have been utilized to reduce the development of white spot lesions during orthodontic treatment: altering dietary habits, providing good oral hygiene and the topical fluoride application (toothpaste, mouth rinse) 8. These approaches, however, rely on patient cooperation, which is unreliable. Furthermore, professional application of fluoride-release agents (fluoride varnish, luting cement and bonding adhesive containing fluoride) can be utilized to minimize biofilm metabolic activity 9. Despite topical fluoride application and brushing technique reinforcement resulting in the alleviation of these lesions, they do not lead to their complete eradication. As a result, research has been conducted to identify new alternative techniques for dealing with similar issues. Adjusting the antibacterial characteristics of a metal surface before appliance installation can effectively deter bacterial infection; many techniques have been used, including direct impregnation with antibiotics, coating and plasma-surface modification. Plasma surface modification (PSM) is a versatile and cost-effective surface treatment method that is gaining popularity in the biomedical industry by the interaction of highly reactive plasma species with the material surfaces, resulting in selectively enhancing surface characteristics while leaving the bulk properties of the materials unaltered. Existing materials may therefore be utilized, and the requirement for new materials can be avoided, reducing the time it takes to build new and better biomedical devices 10. Various gases can be used in plasma activation in plasma surface treatment procedures, such as Ar, CO2, O2, He, H2, and N2. Argon gas has a growing interest in the biomedical field because it is biochemically inert, has a low breakdown voltage, is available and is relatively inexpensive 11. In dentistry, plasma surface modification using Argon gas has many applications, including sterilization 12, increase in bond strength at the dentin/composite interface 13, and improved osseointegration of dental implant 14. the present study is the assessment of antibacterial properties of Argon plasma-treated orthodontic stainless steel brackets against Streptococcus mutans and Lactobacillus acidophilus represented by anti-biofilm and anti-adherence activity and evaluation the effect of change in the duration of plasma treatment exposure on the development of these properties among the three groups of brackets.
.
MATERIALS AND METHODS
A total of 70 orthodontic bicuspid brackets made of stainless steel alloy 316L (International Orthodontic Service IOS™ Roth .022 slot size) were used in the current study. The experimental design of the current research included three main groups, as shown in table (1) &figure (1). Each group contains 20 brackets divided equally into two subgroups for Streptococcus mutans and Lactobacillus acidophilus, respectively. Another group containing 10 treated brackets was used to assess cytotoxicity and biocompatibility, as shown in table (2). At first, to eliminate visible impurities, all brackets were ultrasonicated for 30 minutes in 99 % ethanol and 99.5 % acetone, then dried in a desiccator.

Table 1. The experimental design of the study

Figure 1. 1: Untreated stainless steel bracket 2: stainless steel bracket treated for 15 min. 3: Stainless steel bracket treated for 30 min.

Table 2.The number of brackets and duration of treatment for cytotoxicity assessment
Plasma surface treatment procedure
The plasma surface treatment procedure is done at the Iraqi Ministry of Science and Technology/ Material Research Directorate using the DC magnetron sputtering system. The plasma treatment system used in this study consist of a cylindrical plasma chamber containing two electrodes, the cathode and the anode, separated by 4 cm, providing an electric field for gas discharge. The discharge is carried out with Argon gas at a flow rate of 40 SCCM under the pressure of 5×10-1 mbar. The chamber was evacuated by using a rotary pump (Edward of 12 m³ /h) to vacuum pressure 3×10-1 mbar. The amount of gas inside the chamber is adjusted by using a flow meter, and the pressure inside the chamber was observed by Perani gauge with Edward’s controller 1105. The applied voltage was 500 V which was controlled by a DC power supply. Cathode potential and discharge currents were measured by digital multimeter 15.
In the plasma treatment procedure, the brackets were placed in the magnetron sputtering chamber and distributed in a way that the bonding side faced the anode electrode. High purity argon gas was employed as the ambient gas. The brackets’ bonding side was not treated. The plasma treatment was conducted for 15 min. & 30 min. to produce two main groups of treated brackets with two different periods of exposure, as shown in figure (2).

Figure 2. Plasma surface treatment process of orthodontic stainless steel bracket
Cytotoxicity test
The MTT test is used to assess the biocompatibility of treated stainless steel brackets depending on the capacity of living fibroblasts to produce formazan crystals from (3-(4,5-dimethylthiazote-2yl)-2,5-diphenyl tetrasodium bromide). MTT test was used to evaluate cell viability in response to a treated stainless steel bracket (for three immersion times at 24h, 48h, and 72h). According to the International Standard ISO 10993-part 5 in 2009, At a density of 4 × 10 3 cells per well, Primary human dermal fibroblasts (HDFn) were injected into 96-well plates (Falcon, Teterboro, New Jersey, USA), and incubated for two days at 37°C, in 5% CO 2 in air. After incubation, the culture media was removed, and 200μL of the culture media containing the metal bracket extracts of each immersion period was added. Each well was then dyed with MTT (50 μg/l). The plates were incubated for 4 hours at 37°C with 5% CO 2 in the air. An enzyme-linked immunosorbent assay reader was utilized to determine the degree of light absorption at 570 nm in each well (U2000, Hitachi, Tokyo, Japan). The cell viability tests’ results were given in percentage, the experimental wells’ absorbance at 570 nm compared to the control wells’ at 570 nm. For each subgroup, six replicates were performed 16.
Microbiological aspect
Collection of bacterial samples and culture procedures
Saliva samples were taken from 20 patients undergoing fixed orthodontic treatment (stainless steel brackets) with a duration from starting the treatment not less than two months by spitting into a glass flask; the saliva was then mixed with 1 ml of phosphate-buffered saline (PBS). The samples were sent for microbiological analysis within two hours. In this research, we selected S. mutans and L. acidophilus from the microbial collection because of their significant role in the development of WSL and dental caries; studies suggest that S. mutans has been linked to the onset of dental caries while lactobacilli have a more crucial role in the progression of caries. For S. mutans culturing, 100 ul of the sample was distributed on Mitis Salivarius agar with 0.5 IU/ml bacitracin (Sigma, USA) and then incubated for 48 hours at 37°C in a microaerophilic environment. While L. acidophilus was cultured on Man, Rogosa and Sharpe (MRS) agar for 24h at 37 ⁰C under 95% air and 5% CO2, the isolates were identified based on the colonies’ morphological and cultural features, finally confirmed by Vitek 2.
Adhesion Assay of bacteria to orthodontic brackets
The adhesion ability of S. mutans & L. acidophilius to the brackets was measured by using the plate counting method 17. In this assay, stainless steel bracket segments roughly (1 cm²) were put in tubes containing 5 ml suspension of tested microorganisms which is (1X10^⁸ CFU/mL) according to (0.5 McFarland turbidity standard), then incubated for 1 hour at 37°C. Following incubation, Phosphate buffer saline was used to wash stainless steel brackets three times. The brackets were put in 10 ml fresh PBS and sonicated for 5 minutes at 40 kHz to remove adherent cells. The sonicated PBS was serially diluted and cultivated on tryptic soy agar plates. The viable colony count approach was used to determine the number of adherent bacteria in sonicated PBS (pH= 7.2, 0.1 M), which refers to the degree of adherence of bacteria; this procedure was repeated for each group. The biofilm formation on the orthodontic bracket was tested by using an ELISA plate reader 18. The following steps were applied for six groups in which Serial dilutions of S.mutans and L. acidophilus bacterial cultures were made from nutrient broth to exhibit a cell density of 5 × 106 cells/ ml, and stainless steel bracket (1 cm long) were submerged in 5 ml culture in culture tubes ( for each group), the culture tubes were incubated at 37 °C for 24 hours.
The stainless steel brackets were removed, then rinsed in sterile water, put into culture tubes within 2 ml of nutrient broth, and incubated for 24 hours at 37 °C and 30 °C for S.mutans and L. acidophilus, respectively. Stainless steel brackets segments were removed and rinsed in sterile water before being submerged in tubes within a 0.1% crystal violet solution for 5 min. Stainless steel brackets segments dyed with crystal violet were rinsed in sterile water and moved to separate wells of a 96-well microtiter plate. 25 μl of 30% acetic acid was dropped onto the surface of each catheter to allow solubilization of the adhered biofilm into their respective wells. Absorbance was carried out at 550 nm, and the optical densities of S. mutans and L.acidophilus biofilms on U-C and BCN-C were measured. The optical density was recorded by using 30% acetic acid as a blank. The orthodontic brackets’ adhesion and biofilm formation assays were performed two months after subjecting the brackets to the plasma surface treatment procedure.
Statistical Analysis
The adhesion test results were expressed in viable colonies count attached to bracket surface (CFU), while the biofilm formation test’s results were expressed in optical density (OD). Standard statistical analysis software was used for data assessment (version 20.0, Statistical Package for the Social Sciences, IBM Corporation, Armonk, NY, USA). Descriptive statistics, including mean and standard deviation values, were calculated for the variables. The statistical significance cut-off was set at p ≤ 0.05.
RESULTS
In the current study, four isolates for S. mutans & four isolates for L. acidophilus were obtained to perform microbial and statistical analysis as follows:
Adhesion assay
In the present study, adhesion assay results recorded the highest viable colonies numbers in group 1 in which S.mutans (186±2.8) and L.acidophilus (335± 0.68) when compared with group2 in which the colonies number of S.mutans (168.5±0.70) and L. acidophilus (226±0.01) while the lowest colonies number appeared in group3 in which S.mutans (133±1.00) and L.acidophilus (187±1.00) as shown in figure (3). T-test was used to compare groups 1 &2; groups 1&3 showed a significant difference between group 1 & group 3 at (p ≤ 0.05) as shown in table (3).
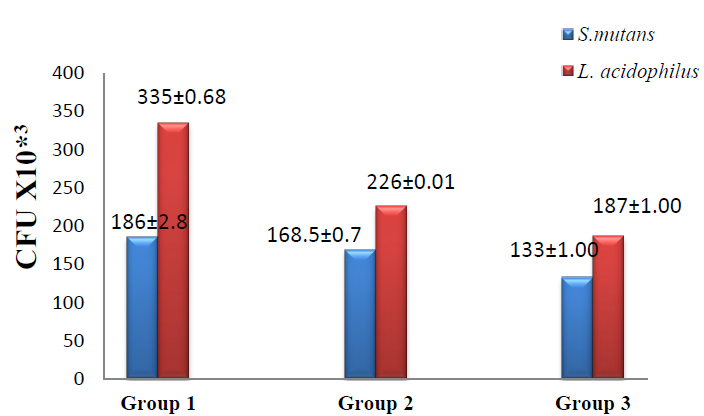
Figure 3. Adhesion assay of S.mutans & L.acidophilus to the brackets in the studied group

Table 3. Adhesion assay of S.mutans & L.acidophilus to the brackets in the studied groups (CFU X10*³ )
Biofilm formation assay
The results of biofilm formation revealed that the lowest value of biofilm formation was recorded in group 3 in which S.mutans (0.274±0.00) and L.acidophilus (0.509±0.00), followed by group2 in which S.mutans (0.443±0.00) and L.acidophilus (0.626±0.01) while the highest value was reported in group1 in which S.mutans (0.495± 0.01) and L.acidophilus (0.688±0.00) as shown in figure (4). T-test was used to compare groups 1 &2, with group 1&3 showing significant differences between groups 1 & 3 at p≤0.05, as shown in table (4).

Figure 4. Biofilm formation assay of S.mutans and L.acidophilus in the studied groups

Table 4. The biofilm formation assay of S.mutans and L.acidophilus in the studied groups
Cytotoxicity test
MTT test on human gingival fibroblast cells in vitro was used to assess the cytotoxicity of the treated brackets. The dose-response viability of fibroblast-like cells treated with the culture media containing treated stainless steel bracket of different immersion periods at (24h, 48h, and 72 h) is expressed in percentage and presented in table (5). MTT test reported more than 80% in both group 2 &group 3 for the three different periods. T-test was done to compare every two groups and revealed a significant difference between 24 and 72 hr, but there is no significant difference between 24 and 48 and between 48 and 72 hr in both group 2 and group 3. However, there is no significant difference between groups 2 &3 during the same periods (p≤ 0.05).

Table 5. MTT assay (cell viability results in percentage at the three immersions time periods (24 hours, 48 hours, and 72 hours).
DISCUSSION
Plasma technologies have become more critical in the medical industry for enhancing the surface characteristics of biomaterials which ultimately affect the cell’s response to the material surface, providing environment-friendly and cost-effectively measures to address the bacterial infection. The present study results indicated reduced bacterial adhesion with lower viable colonies count and higher anti-biofilm activity in plasma-treated stainless steel brackets (group 2 & 3) compared with untreated brackets (group 1) with a statistically significant difference between group1 & 3. These results agree with Shah AG, et al., 19 & Chhattani S et al.,20. The present study’s findings could be attributed to the influence of the plasma treatment on surface chemistry, crystallinity, and ion release. The plasma treatment can induce the development of an oxide layer on the metal surface which can also cause crystallization of amorphous oxide; The anatase crystalline structure has a larger bandgap when compared to the rutile crystalline structure, which increases its surface redox potential to oxidize water molecules to form hydroxyl radicals which play a crucial role in cell interaction21. Komasa S, et al. showed that Argon plasma treatment of titanium surfaces was active enough to cleave C-H bond in the surface layer to form radicals and generate various ROS on the surface, including oxygen-binding compounds and nitrogen; Hydrogen peroxide and hydroxyl radicals are examples of reactive oxygen species (ROS) which are well recognized for causing oxidative damage to cell membranes. The disrupted cell membrane does not have sufficient ability to control substances’ movement through the bacterial wall, which eventually causes cell death 22. For their part, researchers have investigated a range of methods and chemicals that may be used to suppress pathogenic bacteria in the dentistry profession of medicine, including the direct action of Ag and TiO2 nanoparticles on pathogenic bacteria 23.
The release of bactericidal ions from plasma-treated surfaces is another approach to prevent bacterial adhesion. Ions can inhibit bacteria by destroying their envelope and cytoplasmic components, limiting the capacity of peptidoglycans to transport oxygen, inactivating protein enzymatic activities, and/or interrupting DNA replication24. In addition, Plasma treatment can alter the surface hydrophobicity, which significantly affects bacterial attachment and biofilm formation. The Argon plasma treatment of titanium surfaces improved wettability and produced super hydrophilic surfaces22. The S. mutans & L. acidophilus exhibited membrane’s hydrophobic nature 25,26, and the bacteria with the membrane’s hydrophobic nature readily colonize hydrophobic surfaces and vice versa.
Furthermore, plasma treatment has a physical antibacterial effect. Plasma treatment can induce the formation of nanotopography on metal surfaces 27. Nanostructured surfaces or biomimetic surfaces that enable the killing of bacteria by the bacterial membrane’s rupturing arises from the penetration of high aspect ratio nano-features. Hirano M, et al. found that Argon plasma treatment is an effective method for rapidly fabricating antibacterial nanopillars on AISI 316 stainless steel surfaces 28. To evaluate the effect of change in the duration of plasma exposure, two groups of stainless steel brackets were subjected to plasma surface treatment for 15 min. & 30 min. The results indicated reduced bacterial adhesion and higher anti-biofilm activity in plasma-treated stainless steel brackets for 30 minutes (group 3) with a statistically significant difference when compared with group 1, while plasma-treated stainless steel brackets for 15 minutes (group 2) statistically showed no significant difference when compared with group 1 at p≤0.05. These findings could be explained by alteration in plasma discharge parameters (in our study, increasing exposure time will further reduce water contact angle, which ultimately improves surface hydrophilicity and incorporation of more oxygen-containing functional groups onto the treated surface for a longer duration, which strengthens antibacterial properties. These results come in agreement with Lee MJ, et al., 29, who investigated antibacterial properties of titanium surfaces modified by nonthermal atmospheric pressure plasma and reported that a biofilm formation was shown to be lower in samples exposed to plasma for more extended periods when compared with those exposed for shorter periods. The current study evaluated the antimicrobial effects of Argon plasma surface treatment on stainless steel brackets in vitro settings. The limitation of our study is that the test findings may be different in clinically relevant oral circumstances. Seo SH, et al. investigated the antibacterial effect of nonthermal atmospheric pressure plasma in the artificial saliva to imitate some of the in vivo circumstances; the obtained findings suggested that plasma treatment in a natural oral environment with saliva could be more efficient than in some experimental settings 30.
Despite its effectiveness in reducing bacterial adherence and destroying biofilms, the plasma surface treatment can also exhibit a cytotoxicity concern for mammalian cells. In the present study, the MTT test reported cell viability of more than 80% in both group 2 &group 3, as shown in table (5), indicating that the remaining percentage represents the cytotoxic effect of plasma treatment. The cell death could be attributed to reactive oxygen and nitrogen species (RONS) as well as hydrogen peroxide (H2O2) produced by plasma treatment 31. The Increased intracellular RONS is found in both microbial and mammalian cells; however, the antioxidant activity is more significant in specific cell types, which serve to ensure their survival and stimulate cellular proliferation to replenish their numbers throughout time, resulting in differences between cytotoxic effect and antimicrobial effect of plasma treatment 32. Many types of research support minimal tissue damage that can be obtained at specific technical settings (power, gas flow rate). The potential for cell proliferation is enhanced to eliminate biofilm bacteria while minimizing or maintaining a «tolerable» level of tissue cytotoxicity 33. In the present study, an MTT assay measuring the cytotoxicity of orthodontic stainless steel brackets treated with Argon plasma was conducted for three days; depending upon the duration of the orthodontic treatment & the time that stainless steel brackets remain inside the oral cavity, further investigations are required to determine the long-term impact of cellular damage and tissue viability recovery.
CONCLUSION
Plasma surface treatment for orthodontic stainless steel brackets using Argon gas possessed antimicrobial properties against S. mutans and L. acidophilus represented by anti-biofilm and anti-adherence activity with a significant difference between untreated brackets and brackets treated for 30 minutes, indicating that alteration of the time of exposure has an impact on the development of antimicrobial properties which could provide a new preventive measure against white spot lesions development and periodontal problems frequently associated with orthodontic treatment. Further research is needed to assess long-term cellular damage and recovery of cellular viability and determine the most suitable technical parameters of plasma exposure, optimizing antimicrobial properties and making its cytotoxic effect at a negligible level and enhancing tissue viability recovery.
Data Availability: The data used to support this study’s findings are available from the corresponding author.
Funds: By self
Conflict of interest: No conflicts of interest between us or anyone.
Acknowledgments: The authors report that this research was self-funded.
REFERENCES
- Trulsson U, Strandmark M, Mohlin B, Berggren U. A qualitative study of teenagers’ decisions to undergo orthodontic treatment with fixed appliance. Journal of orthodontics. 2002 ;29(3):197-204.
- Julien KC, Buschang PH, Campbell PM. Prevalence of white spot lesion formation during orthodontic treatment. The Angle Orthodontist. 2013 ;83(4):641-7.
- Øgaard B. White spot lesions during orthodontic treatment: mechanisms and fluoride preventive aspects. In Seminars in orthodontics 2008 (Vol. 14, No. 3, pp. 183-193). WB Saunders.
- Tanner AC, Sonis AL, Lif Holgerson P, Starr JR, Nunez Y, Kressirer CA, Paster BJ, Johansson I. White-spot lesions and gingivitis microbiotas in orthodontic patients. Journal of dental research. 2012;91(9):853-8.
- Van Houte J. Role of microorganisms in caries etiology. Journal of dental research. 1994;73(3):672-81.
- Eliades T, Eliades G, Brantley WA. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. American Journal of Orthodontics and Dentofacial Orthopedics. 1995;108(4):351-60.
- Arab S, Malekshah SN, Mehrizi EA, Khanghah AE, Naseh R, Imani MM. Effect of fixed orthodontic treatment on salivary flow, pH and microbial count. Journal of dentistry (Tehran, Iran). 2016;13(1):18.
- Reddy R, Manne R, Sekhar GC, Gupta S, Shivaram N, Nandalur KR. Evaluation of the Efficacy of Various Topical Fluorides on Enamel Demineralization Adjacent to Orthodontic Brackets: An In Vitro Study. The Journal of Contemporary Dental Practice. 2019;20(1):89-93.
- Stecksén-Blicks C, Renfors G, Oscarson ND, Bergstrand F, Twetman S. Caries-preventive effectiveness of a fluoride varnish: a randomized controlled trial in adolescents with fixed orthodontic appliances. Caries research. 2007;41(6):455-9.
- Chu PK, Chen JY, Wang LP, Huang N. Plasma-surface modification of biomaterials. Materials Science and Engineering: R: Reports. 2002;36(5-6):143-206.
- Rodrigues JA, Silva MF, Correia JH. Neutral argon plasma in minimally invasive medical devices for therapy. Multidisciplinary Digital Publishing Institute Proceedings. 2017;1(4):378.
- Whittaker AG, Graham EM, Baxter RL, Jones AC, Richardson PR, Meek G, Campbell GA, Aitken A, Baxter HC. Plasma cleaning of dental instruments. Journal of Hospital Infection. 2004;56(1):37-41.
- Ritts AC, Li H, Yu Q, Xu C, Yao X, Hong L, Wang Y. Dentin surface treatment using a nonthermal argon plasma brush for interfacial bonding improvement in composite restoration. European journal of oral sciences. 2010;118(5):510-6.
- Giro G, Tovar N, Witek L, Marin C, Silva NR, Bonfante EA, Coelho PG. Osseointegration assessment of chairside argon‐based nonthermal plasma‐treated Ca‐P coated dental implants. Journal of Biomedical Materials Research Part A. 2013;101(1):98-103.
- Athab L. Synthesis and Electrical Discharge characterization of Silver nanoparticles in TiO2 thin film by plasma sputtering technique. Ph.D. Thesis, Dep.phys., University of Wasit,Iraq, 2017.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnology annual review. 2005 Jan 1;11:127-52.
- Reid G, Sharma S, Advikolanu K, Tieszer C, Martin RA, Bruce AW. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrobial agents and chemotherapy. 1994;38(7):1490-5.
- Naga V, Nehate SD, Saikumar AK, Sundaram KB. Boron carbon nitride (BCN) nano-coatings of central venous catheters inhibits bacterial colonization. ECS Journal of Solid State Science and Technology. 2020;9(11):115018.
- Shah AG, Shetty PC, Ramachandra CS, Bhat NS, Laxmikanth SM. In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. The Angle Orthodontist. 2011;81(6):1028-35.
- Chhattani S, Shetty PC, Laxmikant SM, Ramachandra CS. In vitro assessment of photocatalytic titanium oxide surface-modified stainless steel and nickel titanium orthodontic wires for its antiadherent and antibacterial properties against Streptococcus mutans. Journal of Indian Orthodontic Society. 2014;48(2):82-7.
- Benčina M, Resnik M, Starič P, Junkar I. Use of plasma technologies for antibacterial surface properties of metals. Molecules. 2021;26(5):1418.
- Komasa S, Kusumoto T, Hayashi R, Takao S, Li M, Yan S, Zeng Y, Yang Y, Hu H, Kobayashi Y, Agariguchi A. Effect of argon-based atmospheric pressure plasma treatment on hard tissue formation on titanium surface. International Journal of Molecular Sciences. 2021;22(14):7617.
- Tahreer Hadi Saleh, Saba Talib Hashim, Salma Nassrullah Malik, and Bahaa Abdullah Laftaah AL-Rubaii, Down-Regulation of flil Gene Expression by Ag Nanoparticles and TiO2 Nanoparticles in Pragmatic Clinical Isolates of Proteus mirabilis and Proteus vulgaris from Urinary Tract Infection. Nano Biomed. Eng., 2019, 11(4): 321-332.
- Ponomarev VA, Orlov EA, Malikov NA, Tarasov YV, Sheveyko AN, Permyakova ES, Kuptsov KA, Dyatlov IA, Ignatov SG, Ilnitskaya AS, Gloushankova NA. Ag (Pt) nanoparticles-decorated bioactive yet antibacterial Ca-and P-doped TiO2 coatings produced by plasma electrolytic oxidation and ion implantation. Applied Surface Science. 2020;516:146068.
- Tahmourespour A, Kasra KR, SALEHI R, NABINEZHAD A. The relationship between cell surface hydrophobicity and antibiotic resistance of streptococcal strains isolated from dental plaque and caries. 2008;10: 251- 255.
- Vadillo-Rodríguez V, Busscher HJ, Norde W, De Vries J, Van Der Mei HC. Dynamic cell surface hydrophobicity of Lactobacillus strains with and without surface layer proteins. Journal of bacteriology. 2004;186(19):6647-50.
- Kovach YE, Zhang F, Gao F, Foster JE. Study of plasma induced nanostructure formation and surface morphology changes on tungsten and stainless steel at atmospheric pressure. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 2019;37(1):011301.
- Hirano M, Hashimoto M, Miura K, Ohtsu N. Fabrication of antibacterial nanopillar surface on AISI 316 stainless steel through argon plasma etching with direct current discharge. Surface and Coatings Technology. 2021;406:126680.
- Lee MJ, Kwon JS, Jiang HB, Choi EH, Park G, Kim KM. The antibacterial effect of nonthermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Scientific reports. 2019;9(1):1-3.
- Seo SH, Han I, Lee HS, Choi JJ, Choi EH, Kim KN, Park G, Kim KM. Antibacterial activity and effect on gingival cells of microwave-pulsed nonthermal atmospheric pressure plasma in artificial saliva. Scientific reports. 2017;7(1):1-1.
- Bekeschus S, Kolata J, Winterbourn C, Kramer A, Turner R, Weltmann KD, Bröker B, Masur K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free radical research. 2014;48(5):542-9.
- Pai KK, Singarapu K, Jacob JD, Madihally SV. Dose dependent selectivity and response of different types of mammalian cells to surface dielectric barrier discharge (SDBD) plasma. Plasma Processes and Polymers. 2015 ;12(7):666-77.
- Suzuki K, Yoshino D. Proliferation-related activity in endothelial cells is enhanced by micropower plasma. BioMed Research International. 2016;2016.
Received: 14 February 2022 / Accepted: 10 March 2022 / Published:15 May 2022
Citation. Ghufran D. Awad, Ihsan D. Awad, Mohammed K. Khalaf, Muhand L. Alshami. In vitro study of the antibacterial effect of plasma surface treatment using Argon gas on orthodontic stainless steel brackets against Streptococcus mutans and Lactobacillus acidophilus . Revis Bionatura 2022;7(2) 48. http://dx.doi.org/10.21931/RB/2022.07.02.48




















