Vol 6 No 4 2021 – 8
INVESTIGATION / RESEARCH
Optimization of cultural conditions affecting improved bioactive metabolite production by endophytic fungus Trichoderma harzianum
Rashid Rahim Hateet1*, Zainab Alag Hassan2, Abdulameer Abdullah Al-Mussawi3 and Shaima Rabeea Banoon4
Available from: http://dx.doi.org/10.21931/RB/2021.06.04.8
ABSTRACT
The present study aimed to optimize cultural conditions for optimum bioactive metabolite production by endophytic fungus Trichoderma harzianum, isolated by surface sterilization method from the leaf of the eucalyptus plant. The fungus was identified based on morphological characterization. Fungal metabolites were carried out by ethyl acetate solvent. The antibacterial activity was tested against Escherichia coli (ATCC 25922) and Staphylococcus aureus (NCTC 6571). Various carbon, nitrogen sources, pH, temperature, incubation period, and NaCl on the antibacterial metabolite production were studied. Bioactive metabolite production of T. harzianum exhibits a broad spectrum of in vitro antibacterial activity against two strains of bacteria. For the optimum production of bioactive metabolites, Dextrose and Glucose were found to be the best sources of carbon and the best sources of Nitrogen Yeast extract (YE) and (NH4)2SO. The maximum production of bioactive metabolites occurs at pH 7 and 25°C.; the NaCl showed a positive influence on bioactive metabolites.
Keywords: Endophytic fungus, Trichoderma harzianum, Optimum conditions, bioactive metabolite, eucalyptus
INTRODUCTION
The genus Trichoderma Pers. consists of species with agricultural, biotechnological, and industrial benefits 1. The first complete genus description date back to 1969 2 and comprised nine species complexes grouping; species morphologically inseparable but genetically diverse. Recent advances in Trichoderma taxonomy have brought the present system of more than two hundred biologic species, supported by the molecular analysis of the variable genomic DNA regions with phylogenetic and taxonomic significance 3. Trichoderma inhabits the root, soil, and foliar environments are highly interactive and free-living fungi and successfully used to control many crop pathogens in field trials 4. Endophytes are microbes, which colonize plants’ internal tissues without causing harmful, apparent adverse effects 5. Complex interactions with host fungi and numerous endophytic fungi are beneficial for their hosts in many ways, including promoting host growth and nutrient gain and enhancing host resistance to phytopathogens, pests or abiotic stress 6. Trichoderma spp. produce secondary metabolites with various biological activities affecting plant metabolism 7. Endophytic fungi are now regarded as an outstanding source of bioactive metabolites because so many of them are cultivated in unusual environments, occupied by millions of unique biological niches (higher plants) 8, 9. Of the 300,000 higher plant species on the earth, one or more endophytes are present in each plant, of the millions which exist here 10. Fungi are an excellent natural source of bioactive secondary metabolites production containing several bioactive agents, including antibiotics, antitumor, antidiabetic, and antioxidants 11,12,13,15,16. The exploration of natural resources to new antimicrobial compounds has become more and more relevant.
Nevertheless, natural products provide important drug sources used in various fields of therapy 17. Auto-regulators administer secondary metabolism by carbon, nitrogen, phosphate sources, trace elements, precursors, secondary metabolism induction enzymes, catabolism suppression and inhibition, and feedback suppression 18. Numerous microbes live in extreme environments, such as high temperatures, high salt concentrations, low pH, and high radiation. Fungal growth and metabolite production also influence some physical factors. The biotechnological production of microorganisms usually depends on their specific environmental adaptations. The production of bioactive and antimicrobial agents, many new and exciting bioactive metabolites such as antibiotics, antivirals, and antioxidants have pharmaceutical, industrial and agricultural importance, isolated and characterized from soil fungi which affected by the physical and chemical parameters such as pH, temperature, incubation period, carbon and nitrogen and amino acid sources 8. The majority of the study indicated a different ecological and cultural factor influencing the biosynthesis of secondary fungal metabolites 19. This study aimed to assess the optimal cultural conditions for producing bioactive metabolites by Trichoderma harzianum endophytic fungus.
MATERIALS AND METHODS
Sample collection
The Euclyptus camaldulensis leaf from the southern province of Maysan in Iraq. 48 hours after collection, The surface sterilization of the leaf was based on 20.
Isolation and characterization of fungi
In Petri dishes containing medium potato dextrose agar (PDA), the segments of surface-sterilized leaf were evenly separated. In the light chamber with 12 hours of light and 12 dark hours, the dishes of Petri were sealed by Parafilm and incubated at 26°C. The Petri dishes were monitored every day to monitor the growth of endophytic fungal colonies from the leaf segments. Identification was made using Rifai’s identification keys 2 and Bissett 21,22,23.
Microbial target organisms
Staphylococcus aureus (NCTC 6571) and Escherichia coli (ATCC 25922) standard test bacteria used in the current study. Cultivation was sub-cultured on a nutrient agar medium before the antibacterial test (Oxoid, England).
Fungal metabolite extraction
The filtrate for fungal culture was extracted three times using a separating funnel with 1:1 (Vol) ethyl acetate. The organic layer has been collected and dehydrated with Na2SO4.
Antibacterial activity
A filter paper disc diffusion technique 24 was used for determining the antibacterial bioactivity of the fungal extract. Petri dishes were prepared, and the bacterial suspension containing 1 x 106 cells per ml of Muller Hinton Agar 25.
Minimal inhibitory concentration.
The standard serial dilution analysis determined the minimum inhibitory concentration (MIC) values 26. The inhibitory test was carried out on the Muller-Hinton agar medium.
Optimization of culture conditions for the production of bioactive metabolite
Basal medium
Potato broth medium with or without was used as a basal medium to determine the optimum conditions for bioactivity exhibited by T.harzianum. Erlenmeyer flasks (500 ml) containing 250 ml Potato broth supplemented with 1 % (w/v) of different carbon or/ nitrogen sources and sterilized.
Effects of carbon sources
Various carbon sources (Dextrose, Galactose, Glucose, Mannose, Starch, and Sucrose) have been amended separately into Tryptic Soy Broth medium at 1% (w/v) using 250 ml of medium in conical flasks. Each flask was inoculated with three discs (5 mm diam) taken from the fungal colony grown on PDA in a Petri dish. Cultures were incubated at 25ºC for 10 days.
Effects of nitrogen sources
Different nitrogen sources (Asparagine, Peptone, Yeast extract (YE), Malt extract (ME), NaNO3, NH4Cl, and (NH4)2 SO3 were separately amended into Tryptic Soy Broth medium at 1% (w/v) using 250 ml of medium in 500 ml flasks. The three discs (5 mm diam) taken from the fungal colony grown on PDA in the petri dish were inoculated. Cultivations have been incubated for 10 days at 25°C.
Effect of pH
Effect pH for detecting the bioactive product metabolite has been tested in the laboratory with liquid cultures containing different pH levels (4,5,6,7,8 and 9).
Effect of temperature
The fungus has been exposed to different temperatures to investigate the best temperature required for the bioactive metabolite (15, 20, 25, 30, and 35°C).
Determination of the incubation period
The impact of the incubation on the active metabolite was calculated using incubation periods ranging from 7 to 26 days.
Effect of NaCl concentration
Incubation in various NaCl concentrations, the effect of salinity on the bioactive metabolite of T.harzianum isolate has been performed, from 3-7% to 1% of carbon and nitrogen sources, while other parameters have remained optimum. The production of bioactive metabolite has been calculated and recorded for each level of sodium chloride.
RESULTS AND DISCUSSION
The present study results showed a significant effect on the bioactive production of T.harzianum metabolites in different microbiological cultural conditions. The production of antibacterial metabolites was determined by the disc diffusion assay method measuring of inhibition zone against two strains of reference bacteria, E. coli (ATCC 25922) and S. aureus (NCTC 6571). Further experiments on cultural optimization conditions to enhance bioactive metabolite production are therefore underway. In the previous decades, more attention has been paid to new bioactive compounds from fungi that have become a natural source 27. Cultural conditions such as pH, temperature, carbon, nitrogen sources, and NaCl concentration were optimized as they affect the metabolite of bioactivity in this study.
Effect of carbon source on antibacterial metabolite for T.harzianum. (Figure 1) Among the carbon sources, dextrose proved to be the best carbon source for antimicrobial metabolites produced by the fungus, with an inhibition zone 18.0 mm against E.coli and 20.0mm against S.aureus. Glucose also gave a similar pattern result followed by Sucrose, Starch, and Mannose, respectively, which agrees with 28. No antibiotic was produced when the medium was supplemented with galactose, and Carbohydrates are known to interfere with the production of secondary metabolites 29. In addition to CO2, water, and energy, the production of intermediates, which produce primary and secondary metabolites is affected by simple carbohydrates like glucose and dextrose through metabolic pathways 30. The addition of glucose resulted in the highest fungal growth but significantly reduced the production of bioactive metabolites. A higher concentration of glucose affects suppressively the production of bioactive metabolites in many fermentation processes 31. It should be noted that the filamentous fungi are ubiquitous organisms able to obtain energy from very different substrates 32.
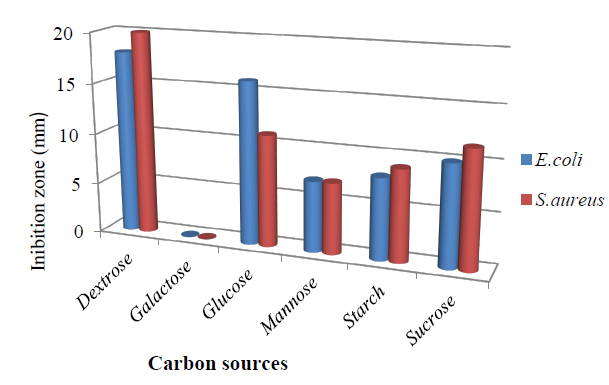
Figure 1. Effect of different carbon sources in the medium on bioactive metabolite by T. harzianum.
Figure 2; shows the effect of various nitrogen sources on T. harzianum production of bioactive metabolites. Maximum antimicrobial activity was obtained when media were supplemented with yeast extract with inhibition zone 18.5mm against E.coli and 20.5 mm against S.aureus followed by (NH4)2SO3, NH4Cl, Asparagine, Peptone, and NaNO3. However, manipulation of nutrient factors has been stated to promote the biosynthesis by microorganisms of secondary metabolites33.
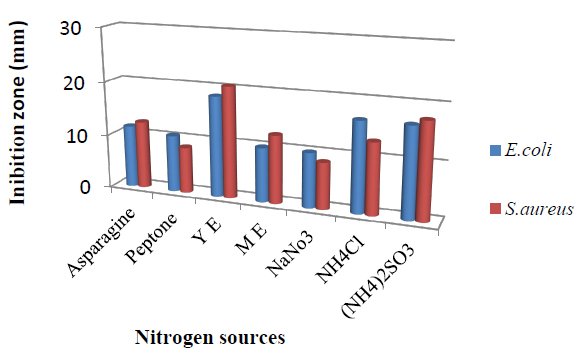
Figure 2. Effect of different nitrogen sources in the medium on bioactive metabolite by T. harzianum.
The effect of pH on antimicrobial metabolite production by the fungus is presented in Figure 3. The optimum pH for antibacterial metabolite production was 7.0 with an inhibition zone of 18.0 mm against E.coli and 16.0 mm against S. aureus; 34 have noted that the most significant number of microorganisms can synthesize pH-based antimicrobial compounds between 5.5 and 8.5.
Figure 3. Effect of pH of the medium on bioactive metabolite by T. harzianum.
The influence of temperature is presented in Figure 4. The T.harzianum, showed a narrow range of bioactive metabolite incubation temperatures. The increase of the incubation temperatures from 25 to 30ºC enhanced bioactive metabolite. Maximum inhibition zone 20.0mm against E.coli and 20.5 mm against S.aureus was recorded at 25C. This indicates the strain was strictly mesophilic for secondary metabolites production. However, the lowest inhibition zone was observed at a low temperature of 15ºC. These results are compatible with 14.
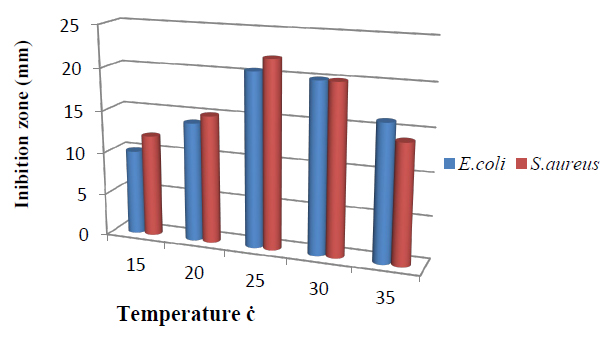
Figure 4. Effect of temperature on bioactive metabolite by T. harzianum.
The influence of the incubation period on bioactive metabolite production of the isolate is presented in Figure 5. The production of metabolite increased and reached its maximum levels after 11 days and after that gradually decreased; this agrees with 14.

Figure 5. Effect of incubation period on bioactive metabolite by T.harzianum.
The influence of NaCl concentration on bioactive metabolite production of the isolate is presented in Figure 6. NaCl concentration of 6g/l was recorded as optimal for bioactive metabolite production.
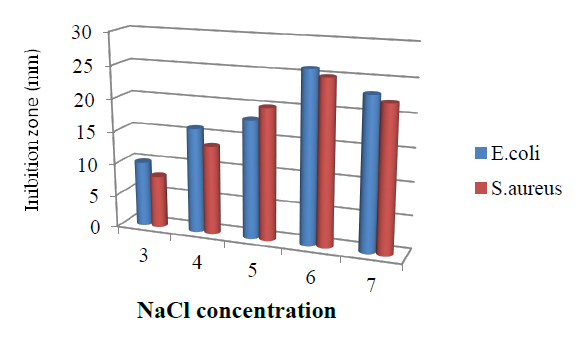
Figure. 6 Effect of NaCl Concentration in the medium on bioactive metabolite by T. harzianum.
CONCLUSION
This study showed good antibacterial activity against gram-positive and gram-negative bacteria produced by T.harzianum grown in optimized conditions.
Acknowledgements
The author would like to express his gratitude to the Department of biology, College of Science, University of Misan, Misan, Iraq.
Conflicts of interest
The author declares that no conflict of interest exists.
REFERENCES
1. Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium, volume 2: Enzymes, biological control and commercial applications. CRC Press; 1998 31 July.
2. Rifai MA. A revision of the genus Trichoderma. Mycological papers. 1969;116:1-56.
3. Bissett J, Gams W, Jaklitsch W, Samuels GJ. Accepted Trichoderma names in the year 2015. IMA fungus. 2015 Dec;6(2):263-95.
4. Reino JL, Guerrero RF, Hernández-Galán R, Collado IG. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochemistry Reviews. 2008 Jan 1;7(1):89-123.
5. White Jr JF, Reddy PV, Bacon CW. Biotrophic endophytes of grasses: a systematic appraisal. Microbial endophytes. New York: Marcel Dekker, Inc. p. 2000 Feb 25:49-62.
6. Rudgers JA, Fischer S, Clay K. Managing plant symbiosis: fungal endophyte genotype alters plant community composition. Journal of Applied Ecology. 2010 Apr;47(2):468-77.
7. Lombardi N, Salzano AM, Troise AD, Scaloni A, Vitaglione P, Vinale F, Marra R, Caira S, Lorito M, d’Errico G, Lanzuise S. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. Journal of Agricultural and Food Chemistry. 2020 May 19;68(27):7246-58.
8. 8. Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and molecular biology reviews. 2003 Dec 1;67(4):491-502.
9. Sharma D, Pramanik A, Agrawal PK. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. 3 Biotech. 2016 Dec;6(2):1-4.
10. Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. Journal of Natural products. 2004 Feb 27;67(2):257-68.
11. Durán-Rivera B, Rojas-Rodas F, Silva-López W, Gómez-Suárez C, Castro-Restrepo D. Molecular identification of Shiitake [Lentinula edodes Berk (Pegler)] and production of secondary metabolites with biotechnological potential. revista bionatura. 2020;5(3): 1183-1188.
12. Hateet RR. GC-MS Analysis of extract for Endophytic fungus Acremonium coenophialum and its Antimicrobial and Antidiabetic. Research Journal of Pharmacy and Technology. 2020 27 January;13(1):119-23.
13. Hateet RR. Evaluation of bioactivity against some pathogenic bacteria and oxidation for fungal secondary metabolites of Fusarium solani isolated from soil. Baghdad Science Journal. 2017;14(3).
14. Ripa EA, Nikkon K, Zaman S, Khondkar P. Optimal conditions for antimicrobial metabolites production from a new Streptomyces sp. RUPA-08PR isolated from Bangladeshi soil. Mycobiology. 2009 1 September;37(3):211-4.
15. Kim HJ, Kim JC, Kim BS, Kim HG, Cho KY. Antibiotic and phytotoxic activities of ophiobolins from Helminthosporium species. The Plant Pathology Journal. 1999 Feb;15(1):14-20.
16. Nicoletti R, Ciavatta ML, Buommino E, Tufano MA. Antitumor extrolites produced by Penicillium species. Int. J. Biomed. Pharm. Sci. 2008;2:1-23.
17. Elaasser MM, Abdel-Aziz MM, El-Kassas RA. Antioxidant, antimicrobial, antiviral and antitumor activities of pyranone derivative obtained from Aspergillus candidus. J. Microbiol. Biotech. Res. 2011;1(4):5-17.
18. Betina V. Bioactive secondary metabolite of microorganisms. Progress in industrial microbiology. 1994;30.
19. Bhattacharyya P, Jha DK. Optimization of cultural conditions affecting growth and improved bioactive metabolite production by a subsurface Aspergillus strain. Int. j. appl. biol. pharm. 2011;2:133-43.
20. Merlin JN, Christhudas IN, Kumar PP, Kumar M, Agastian P. Taxol production by endophytic Fusarium solani LCPANCF01 from Tylophora indica. J Acad Indus Res. 2012;1(5):281.
21. Bissett J. A revision of the genus Trichoderma. I. Section Longibrachiatum sect. nov. Canadian journal of botany. 1984 1 May;62(5):924-31.
22. Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Canadian journal of botany. 1991 Nov 1;69(11):2373-417.
23. Bissett J. A revision of the genus Trichoderma. IV. Additional notes on section Longibrachiatum. Canadian Journal of Botany. 1991 Nov 1;69(11):2418-20.
24. Banoon S, Ali Z, Salih T. Antibiotic resistance profile of local thermophilic Bacillus licheniformis isolated from Maysan province soil. Comunicata Scientiae. 2020 13 July;11:e3921.
25. Gould JC, Bowie JH. The determination of bacterial sensitivity to antibiotics. Edinburgh medical journal. 1952 Apr;59(4):178.
26. Hilário F, Chapla VM, Araujo AR, Sano PT, Bauab TM, Santos LC. Antimicrobial screening of endophytic fungi isolated from the aerial parts of Paepalanthus chiquitensis (Eriocaulaceae) led to the isolation of secondary metabolites produced by Fusarium fujikuroi. Journal of the Brazilian Chemical Society. 2017 Aug;28(8):1389-95.
27. Luzhetskyy A, Pelzer S, Bechthold A. The future of natural products as a source of new antibiotics. Current opinion in investigational drugs (London, England: 2000). 2007 Aug 1;8(8):608-13.
28. Rajput AQ, Khanzada MA, Shahzad S. Effect of different organic substrates and carbon and nitrogen sources on growth and shelf life of Trichoderma harzianum. Journal of Agricultural Science and Technology. 2014 10 July;16(4):731-45.
29. Demain AL, Fang A. Emerging concepts of secondary metabolism in actinomycetes. Actinomycetologica. 1995 Dec 25;9(2):98-117.
30. Turner WB. Fungal metabolites. Fungal metabolites. 1971.
31. Hutter R. Design of culture media capable of provoking wide gene expression. Bioactive Microbial Products: Search and Discovery (Bu’Lock JD, Nisbet LJ, Winstanley DJ, eds.,) Academic, London. 1982:37-50.
32. Kubicek CP, Penttila ME. Regulation of production of plant polysaccharide degrading. Trichoderma And Gliocladium, Volume 2: Enzymes, Biological Control and commercial applications. 1998 31 July;2:49.
33. Kumar SN, Siji JV, Ramya R, Nambisan B, Mohandas C. Improvement of antimicrobial activity of compounds produced by Bacillus sp. associated with a Rhabditid sp.(entomopathogenic nematode) by changing carbon and nitrogen sources in fermentation media. The Journal of Microbiology, Biotechnology and Food Sciences. 2012 1 June;1(6):1424.
34. Thongwai N, Kunopakarn J. Growth inhibition of Ralstonia solanacearum PT1J by antagonistic bacteria isolated from soils in the Northern part of Thailand. Chiang Mai J Sci. 2007;34:345-54.
Received:18 May 2021
Accepted: 30 July 2021
Rashid Rahim Hateet1*, Zainab Alag Hassan2, Abdulameer Abdullah Al-Mussawi3 and Shaima Rabeea Banoon4
1 Department of Biology, College of Sciences, University of Misan, Amarah, Maysan, Iraq. ORCID: 0000-0001-9357-2988
2 College of Nursing, University of Basrah, Basra, Iraq. ORCID: 0000-0003-0322-7414
3 College of Nursing, University of Basrah, Basra, Iraq. ORCID: 0000-0003-2426-5268
4 Department of Biology, College of Science, University of Misan, Maysan, Iraq. ORCID: 0000-0002-9133-2259
* E-mail of the corresponding author: biorashed@uomisan.edu.iq



















