ABSTRACT
Chytridiomycosis is a catastrophic disease currently decimating worldwide amphibian populations, caused by the panzootic chytrid fungus Batrachochytrium dendrobatidis. Massive species decline to extinction catalyzes radical changes in ecosystems globally, including the largest continuous rainforest ecosystem on Earth, the Amazon rainforest. Innovative research that aims to propose feasible mechanisms of mitigation and the origins of the disease is vital, including studies addressing climatic effects on the expansion of chytridiomycosis. Thus, this publication aims to provide a comprehensive review of: i) the current technologies used for B. dendrobatidis detection and monitoring, and ii) the known Neotropical amphibian’s skin microbiota with anti-fungal properties against B. dendrobatidis. Several immunologic and DNA-based methods are discussed to understand the emerging fungal pathogens and their effects on the biosphere, which can help to mitigate the devastating ecological impacts of mass amphibian morbidity. The establishment of rapid and highly accurate B. dendrobatidis detection techniques and methods for monitoring amphibian’s cutaneous microbiome is crucial in the fight against chytridiomycosis.
Keywords: Amphibia; chytrid fungi; skin microbiome; environmental DNA; Next Generation Sequencing.
INTRODUCTION
Chytridiomycosis (hereafter, Chyt) is a devastating fungal disease that affects amphibians and currently contributes to an ongoing loss of biodiversity in frogs, toads, and salamanders. Chyt is caused by the chytrid fungi Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal), which affects both larval and adult stages of amphibian development 1,2. In recent years, amphibian populations and distribution have declined due to various factors, including habitat variations, the introduction of invasive exotic species, unregulated commercial trade, and climate change 3.
Massive amphibian deaths in pristine areas were first observed around 1990. Thirty years later, more than 500 species, or 41% of all amphibians, were considered to be extinct or near-extinction 4-7. As this is a recent phenomenon, studies examining the relationship between environmental change and the emergence of Chyt are needed. Home to many of the world’s amphibians, the Amazon rainforest is of interest because of its immeasurable biodiversity and high susceptibility to climatic changes 8. This review aims to outline current technologies and methods for Bd detection and monitoring. It also provides a comprehensive analysis of known Neotropical amphibians’ skin microbiota, describing specific bacterial strains that produce anti-fungal metabolites inhibiting Bd growth.
This review examines the technologies developed by humans to assess this fungal disease and amphibians’ unique self-defense features. Both contribute to a better understanding of the Chyt disease and the development of novel methods useful for identifying, monitoring, and mitigating the killer fungus.
Environmental controversies worldwide and impacts on Neotropical biodiversity
Amphibians play a pivotal role in ecosystem health due to their regulation of insect populations, which helps control agricultural pests and prevent the transmission of diseases such as dengue, malaria, and Zika virus 4. Amphibians have received further study regarding discovering novel bioactive chemicals for pharmacological applications as new analgesics, antimicrobial agents, and other pharmaceuticals 4,5.
Fungi are one of the largest eukaryotic kingdoms, containing 1.5 to 5 million species that inhabit every biogeographic zone on Earth. These organisms comprise a diverse group with various life cycles, metabolisms, and morphological characteristics 9. Modern fungi evolved from a group of unicellular eukaryotes, and recent studies estimate that the fungal kingdom originated between 710 and 1,060 million years ago in the Precambrian period 10,11. Fungi have a broad impact on Earth’s ecosystems through mutualism, parasitism and commensalism, decomposition, and the synthesis of unique biomolecules 9.
Bd is the best-studied fungal species causing Chyt. As shown in Figure 1, Bd infection begins with the colonization of the host epidermis (Figure 1a), especially in keratin-forming tissue, as occurs in the inguinal region, feet, legs, and the oral disc in the larval stage. The most relevant morphological characteristic of Chytridiomycota fungi is that they produce asexual zoospores with a single flagellum. Generally, Bd has an endobiotic life cycle in which the fungus grows inside the host (Figure 1b). In this instance, the zoospores use their flagellum for motility. Once in contact with the amphibian’s surface epidermis, the zoospores inhabit the skin and develop a germ tube that penetrates the host cell membrane to transfer genetic material. The distal end of the germinal tube then swells and produces a new intracellular chytrid talus and sporangium. This process is repeated, each time burrowing into deeper layers of the host’s epidermis. Once fully penetrated, immature sporangia are transported to the skin surface. When the sporangia finally mature, discharge tubes release mature zoospores into the environment to infect new individuals 6. Bd can also have an epibiotic life cycle (Figure 1c), growing on the surface of the host’s skin, where the zoospores are encysted and form anucleated rhizoids. The cyst matures in a zoosporangium and releases zoospores in a matter of only four days 12. At this time, Chyt disease develops and produces symptoms that include lethargy, depigmentation, or reddening of the skin, and severe cutaneous disorders that result in death 6. Some amphibians are only carriers of the disease and can infect other animal classes, such as fishes 13 and birds 5. However, amphibians are the most vulnerable and suffer from the highest mortality rates.

Figure 1. Bd infection mechanisms. (a) Alytes obstetricans, a small, stocky frog species belonging to Anura, family Alytidae (formerly Discoglossidae), (b) endobiotic cycle, and (c) epibiotic cycle.
Bd has been reported on every continent, except for Antarctica 14. It was shown that Bd originated in Asia, from which multiple epizootic lineages have emerged 15. It is hypothesized that the proliferation of Chyt results from uncontrolled global commerce of amphibians carrying the fungus. The trade of exotic wild animals remains without international regulation, posing an imminent risk that the disease may spread to other regions 16.
The Neotropics consist of a vast region located from Mexico to southern South America, comprising biomes and habitats such as the Andes high-elevation grasslands and the Amazon rainforest 17. The tropical Andes provide ideal Bd growth conditions, with annual temperatures ranging from 22-24 °C 18. Likewise, the Amazon rainforest is characterized by having optimal environmental conditions for fungal growth due to its high relative humidity and tropical temperature 19. Considering that both regions are home to the most extraordinary amphibian species richness globally, Bd’s spread would be devastating 20. It’s presence has already been reported in Amazonian amphibian populations in Colombia, Brazil, and Ecuador 20-22. However, a lack of research still exists studying the prevalence of Chyt in the Amazon rainforest. This does not imply the non-existence of Bd and amphibian populations could be at significant risk.
New developments in Bd identification and monitoring techniques
Management of the disease requires early and accurate detection methods 23,24. Many diagnostic assays are based on PCR techniques, while a few others have focused on antibody and environmental DNA (eDNA) detection. These approaches are summarized in Table 1.
Table 1. Bd identification techniques, types of sampling methods required, and their prevalence efficiency.
PCR-based detection.— Between 1999 and 2005, Bd detection techniques were based on histological analysis 32. These analyses were inadequate for Bd detection because of the long time and high skill required to extract quality tissues 33. In 2005, PCR-based methods began to be implemented, specifically quantitative PCR (qPCR), with a suitable sensitivity to detect Bd 34. Since 2009, other variant PCR techniques like nested-PCR, singlicate-PCR, pooled DNA + qPCR, and Oxford nanopore technology has been developed.
qPCR allows enough sensitivity for Bd detection, and the method of sample collection plays a key role. qPCR samples are collected by a non-invasive sampling technique called «swabbing.» Samples can also be collected in a destructive manner using the tissue extraction method 35,36. In 2006, qPCR was used to detect Bd in Rana subaquavocalis tadpoles from Arizona, USA. Samples of 200 tadpoles were taken by swabbing mouthparts and buccal tissue extraction before DNA isolation 25. qPCR results showed a Bd prevalence of 41 % with destructive sampling methods, while swab samples showed only 21 %. Despite the decreased sensitivity of swab sampling, these results indicate that qPCR can detect Bd in both sampling methods. To improve the Bd detection rate, the authors recommend increasing the number of swab samples per frog to ensure similar reliability to destructive sampling. However, swab samples are useless for qPCR in the early stages of infection 32.
qPCR techniques can detect Bd when the infection level is high. However, it is crucial to detect the infection in its initial stages to manage it 37. Because of this, highly sensitive techniques such as Nested-PCR or qPCR on pooled DNA samples are useful. The sensitivity of Nested-PCR stems from the use of two PCR reactions, with the first reaction amplifying DNA that is then used as a template for a second PCR reaction 38. On the other hand, qPCR on pooled DNA uses the combination of several DNA samples in one, which allows more sensitivity in qualitative studies, where presence/absence is more critical than the amount (that is the case in the initial stages of infection) 39.
In 2014, the effectiveness of qPCR and nested-PCR techniques was compared using Bd zoospores filtered from an aquatic medium inhabited by infected Bombina orientalis frogs, originated from southern China 32. A primary screening revealed that 20 % of the population was Bd-infected. Later, the infected frogs were placed in the same tanks as healthy individuals for six months. Swab samples were collected from ventral skin surfaces 32. Bd analyses were performed by qPCR and nested-PCR. For nested-PCR, 5.8S ribosomal DNA was first amplified using Bd18SF1and Bd28SR1 primers. Then ITS regions were amplified using the Bd1a and Bd2a primers. For qPCR, ITS1-3 Chytr and 5.8S Chytr primers were used. Nucleotide sequences, GC content (%), and melting temperature (°C) of each primer mentioned above are shown in Table 2. qPCR analysis revealed incongruent results in all samples studied. Frogs with positive results in water samples showed negative results on swab samples. The authors claim that these may have been caused by the low concentrations of the collected DNA. On the other hand, nested-PCR obtained congruent results in both samples. Lack of resolution analysis can be resolved by applying another type of sample, such as pooled DNA 40.

Table 2. The Nested-PCR technique used nucleotide sequences, %GC content, and melting temperature of Bd-identification primers. *Primers for Nanopore sequencing.
The size of the pool is an essential parameter in this type of sample. In 2018, Sabino-Pinto et al. analyzed the effectiveness and cost of different pool sizes and loads on Bd detection using qPCR 42. . Researchers inoculated Bd zoospores suspensions in swabs (load sample) and combined them with different amounts of swabs with deionized water (pool size); these combinations are referred to as a pool. Each pool was inserted into a single vial for DNA extraction. The authors also made a cost analysis considering that the cost of DNA extraction of a single sample using a Qiagen kit is 20 € (~22 $), and processing a full qPCR plate costs 52 € (~58 $). So, the cost of processing 10 000 samples individually using Qiagen would be 200 000 € (~223 437 $). If the same number of samples are processed in pools of two and four samples, costs will be 111 000 € (~124 005 $) and 55 000 € (~61 444 $), respectively. Results show that neither the pool size nor the load directly affected detecting Bd’s presence or absence, so using larger pool sizes to reduce costs is possible.
Cost-effective alternatives have been developed by modifying pre-existing PCR protocols. Kriger, Hero & Ashton (2006) developed a modified qPCR protocol denominated singlicate-qPCR 27. This technique is based on a single analysis per sample instead of the three analyses employed by the conventional qPCR procedure. Validation of this protocol was performed in 210 Litoria pearsoniana frogs. Swab samples were extracted from the dorsal, lateral, and ventral surfaces. Conventional qPCR was performed as a control for comparison. For singlicate-qPCR, swab samples were analyzed using the PreqMan Ultra protocol. Results showed a Bd prevalence of 31.7%, while singlicate-qPCR showed 31.5%, differing by only 0.2%. Statistical analysis showed no significant difference (p=0.81) 27.
eDNA-based techniques. — Although swabbing is a non-invasive sampling technique, it requires catching individuals, which can be difficult for small amphibian populations or in inaccessible areas, elevating the sampling effort. However, amphibians and other organism groups leave skin, mucous, and spores that remain on their habitat and where their DNA can be collected. This is eDNA, a resource used to detect organisms’ presence to extract DNA from water, soil, and sediment 43. In their article, Kamoroff and Goldberg (2017) show how eDNA can be used for early Bd detection in the environment and predict fungi outbreaks for amphibians at risk of extinction. Taking lakes water samples and through qPCR, Bd presence was detected on 3 of 7 studied sites. Bd’s frog die-off was observed one month later at the same three sites before mentioned and none on the remaining sites. This study highlights the technique potential for quick decision making in management and conservation strategies 44.
Sieber, Hartikainen and Vorburger (2020) evaluated an eDNA-based method for Bd detection and quantification on large-volume water samples by qPCR. They compared tap and mesocosm water varying spore concentrations. They detected an overall of 87.5% and a high sensitivity reaching to detect 1 spores/L – 100 spores/L, suggesting that false negative can be related to Bd spores’ heterogeneous distributions on water due to encystment on surfaces. Also, they found that it was more likely to detect Bd in tap water samples since 19 of 24 mesocosm water samples showed qPCR inhibition. As an eDNA drawback, samples may also have other compounds that could act as PCR inhibitors. Being that DNA concentration on the water can be low, increasing the volume of water enhances detection probabilities. However, inhibitor concentrations also get increased 28.
Almost all techniques mentioned above are used to detect Bd at the laboratory level, which inevitably requires expensive equipment, refrigeration, costly reagents, and specialized personnel training 45. However, a field-based method is also necessary. Kamoroff, Goldberg and Grasso (2020) followed the rapid detection line testing an in-situ DNA extraction technique using a mobile handled real-time qPCR thermocycler. They performed an experiment with skin swabs and eDNA filtered water samples, contrasting a whole field-based versus a lab-based analysis. Bd was detected on all three sites using swab samples with both lab and field protocols. In contrast, with eDNA samples, the results using both protocols match only on one of the sites. DNA quantification was not successful neither from eDNA nor swab samples using the field protocol. As authors obtain favorable results on Bd detection, they clarify that their field-based method should be improved, and the sensitivity must be increased to avoid false negatives. This study shows how Bd’s presence can be proved on the field within 60 min, with a total cost of $45 29.
DNA and RNA sequencing has reached an unprecedented level of accessibility with the advent of Oxford Nanopore Technologies (ONT), demonstrating its potential in clinical, biosecurity, and environmental analysis. The MinION device’s low-cost equipment, small size, and ease of library preparation powered only by a laptop computer enable the portability for on-site whole genome sequencing (long reads 10-20 Gb of DNA sequence data) and data analysis in the field 46.
The first study on the pathogenic chytrid fungus Bd using a MinION device as high throughput sequencing technology was published in 2018 15. Nanopore generated sequences with almost 99.85% of high-quality referenced data, with primers designed for ribosomal markers targeted to Chytridiomycota 41. They reported the emergence of the Bd pathogen in the early 20th century through mitochondrial genome sequencing, coinciding with the expansion of amphibians’ global commercial trade. East Asia was shown as a geographical hotspot, and intercontinental transmission is ongoing to this day.
Detection by antibodies. — An alternative for easy, quick, and portable Bd detection is immunological techniques such as monoclonal antibodies (mAbs) and polyclonal antibodies (pAbs).
mAbs are specialized glycoproteins produced by the immune system that bind to target molecules with high specificity 47. In 2017, a lateral flow assay (LFA) was developed using the Bd-specific monoclonal antibody 5C4. For development, Bd lineages from Panama, South Africa, Korea, and Mallorca were used to ensure that LFA detected all of them. A solution was prepared containing Bd zoospores and zoosporangia to be injected in BALB/c mice. The antibody test line consisted of purified T-Gel 5C4 with a goat anti-mouse immunoglobulin M class IgM as an internal control; 30. Bd antigen detection by LFA and qPCR was performed in the Chyt animal model Alytes obstetricans using swab sampling and 35 tissues of naturally infected amphibians. The animal model results were negative for control individuals in both tests, qPCR detected four of the five infected individuals, and LFA detected only one. Of the group of samples of naturally infected amphibians, LFA gave positive results for all of eight individuals, in complete agreement with qPCR.
pAbs, meanwhile, are generated by an immune response in which multiple antibodies are produced against a wide variety of structures present in the target molecule 47. In contrast to mAbs, the use of pAbs shows high sensitivity for Bd detection in its initial infection stages. In 2002, the production of pAbs and their introduction in an immunoperoxidase test (IPX) was carried out as a sensitive diagnosis method. The antigen for immunization was obtained using Bd sporangia. The immunization process was carried out in rabbits and sheep, where serum was extracted with Bd antibodies. Sections of Bd culture and Litoria caerulea infected skin sections were used as positive controls, and test sheets incubated with normal serum or pre-bled serum as negative controls. In the IPX test for diagnostic evaluation, 55 fingers of slightly infected L. caerulea and 15 non-infected were used. Results were positive for 34 of 55 samples of infected frogs and 15 of 15 negative results for the control group 31.
Despite the above-mentioned antibody-based techniques, there is another critical factor: Bsal. This is a pathogen closely related to Bd that, although found on some frog species, only develops Chyt clinical signals in salamanders. Bd and Bsal have conserved amino acid sequences. The reason why antibody-based assays cannot differentiate between them is that pAbs can cross-react, and even Bd-specific monoclonal antibody 5C4 binds to proteins from both 30. From this concern, another technique based on in situ hybridization (ISH) has been proved.
RNAScope ISH is a technology designed for amplifying target-specific signals within cells, leaving apart background noise. It is compatible with formalin-fixed, paraffin-embedded tissue specimens and can be performed with chromogenic or fluorescent dyes 48. Ossiboff and their collaborators developed an automated dual-plex chromogenic RNAScope ISH to simultaneously detect and differentiate Bd and Bsal. For this, they made Bd and Bsal cultures using Bd ALKL1 and Bsal AMFP isolates. They designed two target-specific oligonucleotides (ZZ), complementary to Bd JEL 197, and Bsal AMFP 28S rRNA sequences. The assay was performed for both organisms in culture and formalin-fixed paraffin-embedded amphibian tissues, experimentally infected with Bd, Bsal, or both. Those tissues belonged to five salamander and one frog species, preserved in formalin between 2-364 days. Out of 35 trials in total, 33 detected and differentiated correctly Bd and/or Bsal presence, without inconsistencies in the controls. High sensitivity was observed for Batrachochytrium detection in animals with qPCR loads as low as 1.1 × 102 zoospores/microliter 49.
Good management of Bd requires early and fast detection techniques, as described above. Until now, there have been significant efforts to achieve this. However, there is still a need for new methods to deal with this problem. One of the emerging tools for this is the skin microbiome’ study of infected frogs.
ROLE OF THE AMPHIBIAN CUTANEOUS MICROBIOME
Microbiome refers to the habitat formed by microorganisms, their genomes, and the surrounding environment 50. The cutaneous microbiome of amphibians comprises cultivable and non-cultivable microorganisms. The microbiome is vital for protecting amphibians against Bd because of secondary metabolites produced by bacteria 51. These compounds are reported as belonging to a group of anti-fungal metabolites that include 2,4-diacetylphloroglucinol, indole-3-carboxaldehyde and violacein 53 (Figure 2).
Figure 2. Anti-fungal metabolites (a) 2,4-diacetylchlorchlorucinol produced by Lysobacter gummosus, (b) indole-3-carboxaldehyde and (c) violacein produced by Janthinobacterium lividum.
Bioassays are useful to determine the anti-fungal properties by studying the effect of these compounds on Bd’s growth rate. Only two independent studies, Catenazzi et al. (2018) in Peru 54, and Bresciano et al. (2015) in Ecuador 21 characterized the cultivable skin-bacterial diversity of amphibians from the tropical Andes. Interestingly, bacteria belonging to the genera Janthinobacterium, Pseudomonas, and Serratia were found in both countries. Their results indicate that the cutaneous microbiome components’ inhibitory effects could correspond to bacterial anti-fungal metabolites instead of competition for resources between bacteria and Bd fungus.
Catenazzi et al., (2018) isolated cultivable bacteria from eight frog species and analyzed their Bd inhibition potential 54. Results showed that Pseudomonas entomophila was the bacteria with the highest inhibition probability (78.04 %), while other non-identified species of this same genus showed a 55.96 % probability of inhibition. Cultivable bacterial diversity isolated from the cutaneous microbiome is as follows: Pseudomonas (55 %) > Paenibacillus (10 %) = Rahnella (10 %) = Serratia (10 %) > Aeromonas (5 %) = Janthinobacterium (5 %) = Sphingobacterium (5 %). Unfortunately, there were no studies conducted regarding the cutaneous microbiome of Amazon amphibians. Therefore, the scope of the problem and the biodiversity of autochthonous anti-Bd bacteria are yet unknown. Figures 3 and 4 show the prevalence of cultivable bacterial diversity found in several Andean’s cutaneous microbiome amphibian species, which diverge in temporality despite being in the same geographical region 55. Pseudomonas is the most common genus isolated from all frog species studied. Gastrotecha excubitor reports the highest biodiversity of cultivable bacteria recorded to date. This finding explains why G. excubitor is the most resistant species to Bd-infection, suggesting a relationship between Chyt disease resistance and the bacterial biodiversity associated with its cutaneous microbiome 55.
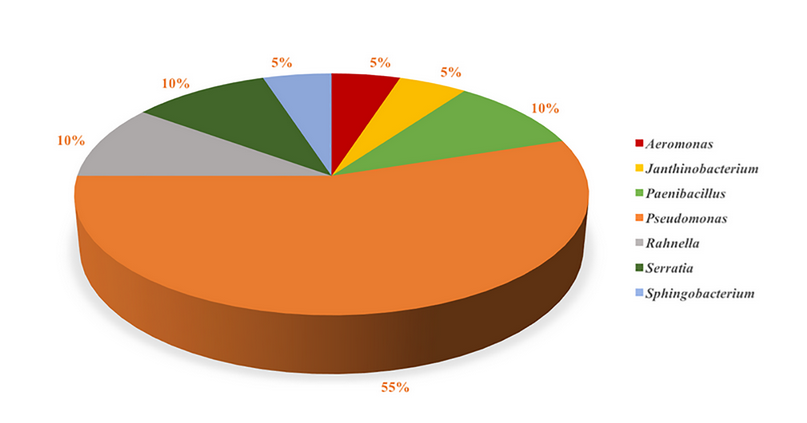
Figure 3. Distribution of anti-Bd bacterial genus isolated from the cutaneous microbiome of Andean amphibian species (Bresciano et al., 2015; Catenazzi et al., 2018).
It is also essential to consider that a fraction of the bacteria associated with the amphibian cutaneous microbiome is non-cultivable. Walke et al. (2011) proposed that almost 37% of the total bacterial diversity found in an amphibian’s skin is non-cultivable 56. Bates et al. (2018) used 16S rRNA metabarcoding to study the bacterial skin community in Alytes obstetricans, native to the Pyrenees, on diverse Bd infection types during different life stages 57. In addition to uncovering the host bacterial population, they revealed that infection dynamics and the individual’s life stage are essential factors in bacterial skin communities’ configuration. Rebollar et al. (2018) used 16S rRNA gene amplicon sequencing and described the taxonomic composition of the cutaneous microbiome associated with Neotropical frog Craugastor fitzingeri, discovering Pseudomonas as the main component 58. These results demonstrate the effectiveness of the metagenomics tools in revealing the skin microbiome’s functions and determining their defense role against fungal pathogens.

Figure 4. Prevalence of cultivable bacterial biodiversity found in the cutaneous microbiome of Andean amphibians. Note that the high prevalence of Pseudomonas genus is found in most frog species studied (in green color) and that Gastrotecha excubitor species have more cultivable bacterial biodiversity.
On the other hand, not all bacteria living on amphibian skin are beneficial for Bd resistance 59. In vitro tests showed that the Microbacterium genus could promote Bd growth since they can produce nutritive compounds for the pathogenic fungus. Thus, if Microbacterium is found in a more significant proportion in the amphibian’s microbiome, it could help advance a developing Bd infection 59.
Some controversy exists as to whether cutaneous microbiome composition or the secretion of antimicrobial peptides is the amphibian Bd defense’s main factor. A comparison between Bd-susceptible species Gastrotheca nebulanastes and the resistant specie G. excubitor was carried out in 2017 55. Inhibitory potentials of antimicrobial peptides secreted by both species showed no significant differences in anti-Bd activity 55. However, G. excubitor exhibited higher richness and abundance of anti-Bd bacteria in its cutaneous microbiome, which was the differentiating factor between the species. Contrarily, other authors described both antimicrobial peptides and the cutaneous microbiome as equally contributing to Andean amphibian species’ survival 60. To better understand amphibians’ applied resistance mechanisms against Bd, a clear need exists for other multi-, inter-, and transdisciplinary studies where numerous factors are considered.
CONCLUDING REMARKS
Various Bd identification and monitoring techniques are evaluated and critiqued. The mAb test cannot provide positive results when Bd is in a basal state, which is a problem because early control measures are needed to decrease amphibian morbidity. In contrast, pAb offers high sensitivity for Bd detection in its initial infection stages. RNAScope technology allows the detection and differentiation of Bd and Bsal presence, but histological procedures and image analysis are still key challenges. Recent studies revealed that qPCR and nested-PCR are efficient techniques in different conditions. However, their use is limited because a large number of samples are required. qPCR can be used with a high quantity of swab samples, while nested-PCR allows the detection of Bd when the infection level is low. A viable alternative could be the use of nested-PCR for analysis of filtered water samples inhabited by frogs because this technique allows Bd detection even at low levels. Another option is to use pooled DNA samples with qPCR techniques since neither the load nor the pool size affects detectability. Hence, it is possible to pool several samples without the loss of resolution analysis and reducing costs.
The development of novel technologies allows reducing analytical costs while maintaining an acceptable level of sensitivity. Oxford Nanopore Technology sequencing distinguishes itself as a powerful high-throughput technology for in-field, real-time epidemiological studies focused on identifying emerging fungal pathogens in the wild. Furthermore, the development of new techniques using eDNA allows BD detection without even collecting study’ individuals. In the last year, no effort has been spared to take the laboratory to the field, for which portable equipment such as real-time qPCR has been developed, which gives a perspective of how the analysis will look like in the future.
Amphibians use different strategies for resistance and resilience in fighting Bd infection. Secondary metabolites produced by amphibian skin-bacteria are suggested as adequate biological controls of the killer Bd fungus. For this reason, in-depth studies of autochthonous anti-Bd bacteria for the prevention and/or mitigation of Chyt are proposed. Assessment of the Pseudomonas genus is highly recommended because of its high prevalence and Bd-inhibition rate. The identification and evaluation mechanisms and the analysis of the skin microbiome reviewed in this article contribute to a better understanding of Chyt devastating disease caused by the Bd fungus and the resistance and resilience mechanisms used by amphibians for survival.
Abbreviations. — Chyt: Chytridiomycosis; Bd: Batrachochytrium dendrobatidis; Bsal: Batrachochytrium salamandrivorans; PCR: polymerase chain reaction; ONT: Oxford Nanopore Technologies; pAbs: polyclonal antibodies; mAbs: monoclonal antibodies; LFA: lateral flow assay; qPCR: quantitative PCR.
Acknowledgments. — We thank J. Luzuriaga for his technical and artistic assistance in the handmade drawing of Figure 1. Also, thanks to A. Naranjo and J. Kabir for their valuable grammatical support.
Conflict of interest. —The authors declare no conflict of interest.
Funding. — No source of funding.
REFERENCES
1 Bradley, P. W. et al. Shifts in temperature influence how Batrachochytrium dendrobatidis infects amphibian larvae. PloS one 14, e0222237-e0222237, doi:10.1371/journal.pone.0222237 (2019).
2 Martel, A. et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences 110, doi:10.1073/pnas.1307356110 (2013).
3 Fisher, M. & Garner, T. The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21, 2-9, doi:10.1016/j.fbr.2007.02.002 (2007).
4 De Sá, R. O. Crisis global de biodiversidad: importancia de la diversidad genética y la extinción de anfibios. Agrociencia 9, 513 (2005).
5 Hanlon, S. M., Henson, J. R. & Kerby, J. L. Detection of amphibian chytrid fungus on waterfowl integument in natural settings. Diseases of aquatic organisms 126, 71-74 (2017).
6 Piotrowski, J. S., Annis, S. L. & Longcore, J. E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9-15 (2004).
7 Scheele, B. C. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459-1463, doi:10.1126/science.aav0379 (2019).
8 Whitworth, A., Villacampa, J., Serrano Rojas, S. J., Downie, R. & MacLeod, R. Methods matter: Different biodiversity survey methodologies identify contrasting biodiversity patterns in a human modified rainforest — A case study with amphibians. Ecological Indicators 72, 821-832, doi:https://doi.org/10.1016/j.ecolind.2016.08.055 (2017).
9 Choi, J. & Kim, S.-H. A genome Tree of Life for the Fungi kingdom. Proceedings of the National Academy of Sciences 114, 9391-9396, doi:10.1073/pnas.1711939114 (2017).
10 Watkinson, S. C. in The Fungi Vol. 3 (ed Sarah C. Watkinson) 89-203 (Elsevier, 2016).
11 Naranjo-Ortiz, M. A. & Gabaldón, T. Fungal evolution: cellular, genomic and metabolic complexity. Biological Reviews 000, 1-35, doi:10.1111/brv.12605 (2020).
12 James, T. Y. et al. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: Lessons from the first 15 years of amphibian chytridiomycosis research. Ecology and Evolution 5, 4079-4097, doi:10.1002/ece3.1672 (2015).
13 Liew, N. et al. Chytrid fungus infection in zebrafish demonstrates that the pathogen can parasitize non-amphibian vertebrate hosts. Nature Publishing Group 8, 1-10, doi:10.1038/ncomms15048 (2017).
14 Miller, C. A. et al. Distribution modeling and lineage diversity of the chytrid fungus Batrachochytrium dendrobatidis ( Bd ) in a central African amphibian hotspot. PloS one 13, 1-16 (2018).
15 O’hanlon, S. J. et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360, 621-627 (2018).
16 Kolby, J. E. & Daszak, P. The Emerging Amphibian Fungal Disease, Chytridiomycosis : A Key Example of the Global Phenomenon of Wildlife Emerging Infectious Diseases. Emerging Infections 4, 1-17, doi:10.1128/microbiolspec.EI10-0004-2015.Correspondence (2016).
17 Antonelli, A. et al. Amazonia is the primary source of Neotropical biodiversity. Proceedings of the National Academy of Sciences 115, 6034, doi:10.1073/pnas.1713819115 (2018).
18 Cabrera-condarco, W. H. Diversidad florística de un bosque montano en los Andes tropicales del noroeste de Bolivia. Ecología en Bolivia 40, 380-395 (2005).
19 Pacheco, A. M. & Scussel, V. M. Selenium and aflatoxin levels in raw Brazil nuts from the Amazon basin. Journal of agricultural and food chemistry 55, 11087-11092, doi:10.1021/jf072434k (2007).
20 Becker, C. G., Rodriguez, D., Lambertini, C., Toledo, L. F. & Haddad, C. F. B. Historical dynamics of Batrachochytrium dendrobatidis in Amazonia. Ecography 39, 954-960, doi:10.1111/ecog.02055 (2016).
21 Bresciano, J. C. et al. Variation in the Presence of Anti-Batrachochytrium dendrobatidis Bacteria of Amphibians Across Life Stages and Elevations in Ecuador. EcoHealth 12, 310-319, doi:10.1007/s10393-015-1010-y (2015).
22 McCracken, S., P. Gaertner, J., Forstner, M. & Hahn, D. Detection of Batrachochytrium dendrobatidis in amphibians from the forest floor to the upper canopy of an Ecuadorian Amazon lowland rainforest. Herpetological review 40, 190-195 (2009).
23 Rooij, P. V., Martel, A., Haesebrouck, F. & Pasmans, F. Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Veterinary Research 46, 137, doi:10.1186/s13567-015-0266-0 (2015).
24 Walker, S. F. et al. Environmental detection of Batrachochytrium dendrobatidis in a temperate climate. Diseases of aquatic organisms 77, 105-112, doi:10.3354/dao01850 (2007).
25 Retallick, R. W. R., Miera, V., Richards, K. L., Field, K. J. & Collins, J. P. A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Diseases of Aquatic Organisms 72, 77-85, doi:10.3354/dao072077 (2006).
26 Shin, J., Bataille, A., Kosch, T. A. & Waldman, B. Swabbing often fails to detect amphibian chytridiomycosis under conditions of low infection load. PLoS One 9, e111091, doi:10.1371/journal.pone.0111091 (2014).
27 Kriger, K. M., Hero, J. M. & Ashton, K. J. Cost efficiency in the detection of chytridiomycosis using PCR assay. Diseases of Aquatic Organisms 71, 149-154, doi:10.3354/dao071149 (2006).
28 Sieber, N., Hartikainen, H. & Vorburger, C. Validation of an eDNA-based method for the detection of wildlife pathogens in water. Dis Aquat Organ 141, 171-184, doi:10.3354/dao03524 (2020).
29 Kamoroff, C., Goldberg, C. & Grasso, R. Rapid detection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) using in-situ DNA extraction and a handheld mobile thermocycler. Authorea, doi:10.22541/au.159363453.33681758 (2020).
30 Dillon, M. J. et al. Tracking the amphibian pathogens Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans using a highly specific monoclonal antibody and lateral-flow technology. Microbial biotechnology 10, 381-394, doi:10.1111/1751-7915.12464 (2016).
31 Berger, L. et al. Production of polyclonal antibodies to Batrachochytrium dendrobatidis and their use in an immunoperoxidase test for chytridiomycosis in amphibians. Diseases of Aquatic Organisms 48, 213-220 (2002).
32 Shin, J., Bataille, A., Kosch, T. A. & Waldman, B. Swabbing often fails to detect amphibian chytridiomycosis under conditions of low infection load. PLoS One 9, e111091, doi:10.1371/journal.pone.0111091 (2014).
33 Berger, L., Speare, R. & Kent, A. Diagnosis of chytridiomycosis in amphibians by hystological examination. Zoos’ Print Journal 15, 184-190, doi:10.11609/JoTT.ZPJ.15.1.184-90 (1999).
34 Annis, S. L., Dastoor, F. P., Ziel, H., Daszak, P. & Longcore, J. E. A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. Journal of Wildlife Diseases 40, 420-428, doi:10.7589/0090-3558-40.3.420 (2004).
35 Clare, F., Daniel, O., Garner, T. & Fisher, M. Assessing the ability of swab data to determine the true burden of infection for the amphibian pathogen Batrachochytrium dendrobatidis. EcoHealth 13, 360-367, doi:10.1007/s10393-016-1114-z (2016).
36 Waddle, A. W. et al. Systematic approach to isolating Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 127, 243-247, doi:10.3354/dao03203 (2018).
37 Bataille, A. et al. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Molecular Ecology 22, 4196-4209, doi:10.1111/mec.12385 (2013).
38 Müller, H.-J. & Prange, D. R. in PCR – Polymerase-Kettenreaktion (eds Hans-Joachim Müller & Daniel Ruben Prange) 85-87 (Springer Spektrum, 2016).
39 Hellicar, A. D., Rahman, A., Smith, D. V. & Henshall, J. M. Machine learning approach for pooled DNA sample calibration. BMC Bioinformatics 16, 214, doi:10.1186/s12859-015-0593-1 (2015).
40 Sabino-Pinto, J., Martel, A., Pasmans, F., Steinfartz, S. & … Pooling skin swabs does not inhibit qPCR detection of amphibian chytrid infection. PloS one 14, e0214405 (2019).
41 Wurzbacher, C. et al. Introducing ribosomal tandem repeat barcoding for fungi. Molecular Ecology Resources 19, 118-127, doi:10.1111/1755-0998.12944 (2019).
42 Sabino-Pinto, J. et al. Detectability vs. time and costs in pooled DNA extraction of cutaneous swabs: A study on the amphibian chytrid fungi. Amphibia Reptilia 40, 29-39, doi:10.1163/15685381-20181011 (2019).
43 Chestnut, T. et al. Heterogeneous occupancy and density estimates of the pathogenic fungus Batrachochytrium dendrobatidis in waters of North America. PloS one 9, e106790-e106790, doi:10.1371/journal.pone.0106790 (2014).
44 Kamoroff, C. & Goldberg, C. S. Using environmental DNA for early detection of amphibian chytrid fungus Batrachochytrium dendrobatidis prior to a ranid die-off. Dis Aquat Organ 127, 75-79, doi:10.3354/dao03183 (2017).
45 Ghosh, P. N., Fisher, M. C. & Bates, K. A. Vol. 9 (2018).
46 Runtuwene, L., Tuda, J., Mongan, A. & Suzuki, Y. in Single Molecule and Single Cell Sequencing. Vol. 1129 (ed Suzuki Y) 143-150 (Advances in Experimental Medicine and Biology. Springer, 2019).
47 Machado, N. P., Téllez, G. A. & Castaño, J. C. Anticuerpos monoclonales: desarrollo físico y perspectivas terapéuticas. Infectio 10, 186-197 (2006).
48 Wang, F. et al. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. Journal of Molecular Diagnostics 14, 22-29, doi:10.1016/j.jmoldx.2011.08.002 (2012).
49 Ossiboff, R. J. et al. Differentiating Batrachochytrium dendrobatidis and B. salamandrivorans in Amphibian Chytridiomycosis Using RNAScope® in situ Hybridization. Frontiers in Veterinary Science 6, 1-10, doi:10.3389/fvets.2019.00304 (2019).
50 Marchesi, J. R. & Ravel, J. The vocabulary of microbiome research: a proposal. Microbiome 3, 31-31, doi:10.1186/s40168-015-0094-5 (2015).
51 Loudon, A. H. et al. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Frontiers in microbiology 5, 441-441, doi:10.3389/fmicb.2014.00441 (2014).
52 Brucker, R. M. et al. The identification of 2,4-diacetylphloroglucinol as an anti-fungal metabolite produced by cutaneous bacteria of the salamander plethodon cinereus. Journal of Chemical Ecology 34, 39-43, doi:10.1007/s10886-007-9352-8 (2008).
53 Brucker, R. M. et al. Amphibian chemical defense: Anti-fungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. Journal of Chemical Ecology 34, 1422-1429, doi:10.1007/s10886-008-9555-7 (2008).
54 Catenazzi, A. et al. Widespread Elevational Occurrence of Antifungal Bacteria in Andean Amphibians Decimated by Disease: A Complex Role for Skin Symbionts in Defense Against Chytridiomycosis. Frontiers in microbiology 9, 465-465, doi:10.3389/fmicb.2018.00465 (2018).
55 Burkart, D., Flechas, S. V., Vredenburg, V. T. & Catenazzi, A. Cutaneous bacteria, but not peptides, are associated with chytridiomycosis resistance in Peruvian marsupial frogs. Animal Conservation 20, 483-491, doi:10.1111/acv.12352 (2017).
56 Walke, J. B., Harris, R. N., Reinert, L. K., Rollins-Smith, L. A. & Woodhams, D. C. Social Immunity in Amphibians: Evidence for Vertical Transmission of Innate Defenses. Biotropica 43, 396-400, doi:10.1111/j.1744-7429.2011.00787.x (2011).
57 Bates, K. A. et al. Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure. Nature Communications 9, 693-693, doi:10.1038/s41467-018-02967-w (2018).
58 Rebollar, E. A. et al. The skin microbiome of the neotropical frog Craugastor fitzingeri: Inferring potential bacterial-host-pathogen interactions from metagenomic data. Frontiers in Microbiology 9, 466, doi:10.3389/fmicb.2018.00466 (2018).
59 Becker, M. H. et al. Phylogenetic distribution of symbiotic bacteria from Panamanian amphibians that inhibit growth of the lethal fungal pathogen Batrachochytrium dendrobatidis. Molecular ecology 24, 1628-1641, doi:10.1111/mec.13135 (2015).
60 Flechas, S. V. et al. Microbiota and skin defense peptides may facilitate coexistence of two sympatric Andean frog species with a lethal pathogen. The ISME Journal 13, 361-373, doi:10.1038/s41396-018-0284-9 (2019).
Received: 13 October 2020
Accepted: 10 November 2020
Génesis L. Romero-Zambrano1, Stalin A. Bermúdez-Puga1, Alex F. Sánchez-Yumbo1, Jomira K. Yánez-Galarza1, H. Mauricio Ortega-Andrade2,3, and Leopoldo Naranjo-Briceño1,4,*
1Biotechnology Engineering Career. Faculty of Life Sciences. Universidad Regional Amazónica Ikiam, Tena, Ecuador 150150
2Biogeography and Spatial Ecology Research Group, Universidad Regional Amazónica Ikiam, Tena, Ecuador 150150
3Herpetology Division, Instituto Nacional de Biodiversidad (INABIO), calle Rumipamba 341 y Av. de los Shyris, Quito, Ecuador.
4Applied Microbiology Group, Universidad Regional Amazónica Ikiam, Tena, Ecuador 150150
*Corresponding author. Email: leopoldo.naranjo@ikiam.edu.ec
ORCID:
Génesis Romero Zambrano: https://orcid.org/0000-0003-0928-7477
Stalin Bermúdez Puga: https://orcid.org/0000-0002-2375-0491
Alex Sánchez Yumbo: https://orcid.org/0000-0001-9636-8069
Jomira Yánez Galarza: https://orcid.org/0000-0003-2816-9759
Mauricio Ortega Andrade: https://orcid.org/0000-0001-9464-3688
Leopoldo Naranjo-Briceño: https://orcid.org/0000-0003-4838-0818

























