Vol 6 No 1 2021 – 11
NVESTIGATION / RESEARCH
Optimization of the micropropagation of elite adult trees of Sequoia sempervirens: forest species of interest in the Basque Country, Spain
Available from: http://dx.doi.org/10.21931/RB/2021.01.01.11
ABSTRACT
Forest trees are renewable sources of timber and other valuable non-timber products. Nowadays, the increase in population and demand for forestry products results in overexploitation of forestry. Therefore, there is an urgent need to produce elite plants with higher productivity under stress derived from climate change to have available to afforestation. For this reason, propagation methods should be improved to be more efficient in terms of quality and productivity.
The main species planted in the Basque Country is Pinus radiata; during the last three years, Pinus radiata plantations have suffered a fungus attack affecting mainly needles until the tree’s death. This crisis is caused by the combined action of two fungi of the genus Dothistroma and Lecanosticta acicola, whose expansion seems to have been enhanced by weather-related factors, such as humid and hot summers. Although we have evidence of this disease’s presence in our mountains since 1942, the disease has had a speedy expansion with an aggressive effect for reasons that are not scientifically known today. For the above, Basque Country forestry sector is looking for alternative species to be used in its plantations. Part of the forestry sector considers that Sequoia sempervirens could be a good choice for plantations. Besides, its high-quality wood and its tolerance to the attack of several pathogens and other diseases derived from climate change are characteristics that could confer some advantage over other forest species.
The main goal of this study was to optimize the micropropagation of adult elite trees of Sequoia sempervirens. The effect of 6-benzylaminopurine, meta-Topolin and Kinetin, and 4 types of explant in the multiplication stage were analyzed to carry out this objective. Furthermore, the effect of two types of auxins: 1-naphthalene acetic acid, indole-3-butyric acid, and a mixture of both, were evaluated on the induction of roots and their subsequent effect on the acclimatization process. The best multiplication index was obtained when 4.4 µM 6-benzylaminopurine and apical explants longer than 1.5 cm of length were used. The root induction percentage was 75% in the most responsive genotype analyzed when 4.4 µM 6-benzylaminopurine was used on the induction stage, and 50 µM of 1-naphthalene acetic acid was used for rooting. Finally, after 3 months in the greenhouse, the explants cultured with Kinetin and rooted in a culture medium with indole-3-butyric acid showed the highest acclimatization success (94%).
Keywords: acclimatization, auxins, cytokinins, multiplication index, organogenesis, rooting, shoot induction.
INTRODUCTION
The Coast redwood or California redwood Sequoia sempervirens (D. Don.) Endl. (Taxodiaceae) is a valuable forest species and occurs naturally in Western North America, especially in California1,2. This species is the tallest tree on earth with a high volume of standing biomass, in some stands exceeding 3500 metric tons/hectare3.
This conifer has been introduced and domesticated in European countries such as Romania, Spain, France, Great Britain, Russia, and Turkey, and it can be used for reforesting due to the quality of its wood and for ornamental purposes. In Spain, this species has been used by foresters for its productivity, its tolerance to the attack of several pathogens and other diseases. Moreover, its reforestation is recommended on valley bottoms and fotthills4.
Nowadays, redwood shows seed reproduction difficulties, displaying low germination and rooting rates, dormancy of the shoot, and low seedling viability 5,6. Therefore, biotechnological in vitro techniques of plant tissue culture as micropropagation, organogenesis, adventitious or axillary shoot/bud regeneration, shoot tip culture, micrografting, and somatic embryogenesis emerged as useful tools for the propagation and conservation of germoplasm7,8,9.
There are few investigations on micropropagation in the specific case of Sequoia sempervirens, either through organogenesis or somatic embryogenesis. Arnaud et al.10 developed a protocol for micropropagation and rejuvenation of the species using direct organogenesis and somatic embryogenesis. Years later, Mihaljević et al.1 investigated the root formation in micropropagated shoots of Sequoia sempervirens using Agrobacterium. In the 21st century, Korban and Soul11 developed a procedure for the micropropagation of juvenile and adult material, and Lui et al.12 worked on the shoot regeneration and somatic embryogenesis from needles of redwood. The medium-term conservation of Sequoia sempervirens was investigated by Ozudogru et al.6, and Meneguzzi et al.2 evaluated shoot multiplication of two Sequoia sempervirens genotypes with the addition of Kinetin.
Considering all the information mentioned above, our study's objective was to optimize the micropropagation process of elite selected adult trees of Sequoia sempervirens. We focused on
improving 1) the multiplication phase using different types of explants and cytokinins, 2) the rooting using different auxins, and the subsequent acclimatization phase under
ex vitro conditions.
MATERIALS AND METHODS
Plant material
Stem sections were collected from the basal parts of three different mother trees (7, 11 and 12) of Sequoia sempervirens located in Ataun, Gipuzkoa, Basque Country (Spain), at the geographic coordinates: 42° 58′ 41″ N and 2° 10′ 53′′ O.
Shoot induction
In vitro micro shoots growing in half-strength ARN medium (Arnaud et al.10) were selected to carry out the experiments. Four types of explants were used: A) apical explants of 1.5 cm in length (AG), B) apical explants of 1.0 cm length (AP), C) basal explants of 1.5 cm length (BG), and D) basal explants of 1.0 cm in length (BP). Explants were cultured in glass jars (Merck) with 25 mL of ARN multiplication medium supplemented with 3% sucrose and 8 gL-1 Difco Agar®. The effect of different cytokinins for the induction of shoots was evaluated: A) 6-benzylaminopurine (BAP), B) meta-Topolin (m-T), and D) kinetin (K) at a concentration of 4.4 µM. The pH of the medium was adjusted to 5.8 before autoclaving (121°C, 20 min). The explants were cultivated in the different induction media for four weeks in the growth chamber at a temperature of 21 ± 1°C, under 16-h photoperiod with 120 µmol m-2 s-1 of light intensity cool white fluorescent tubes (TLD 58 W/33; Philips, France).
Shoot elongation
The explants induced in the multiplication phase were cultivated for six weeks in shoot elongation medium. This medium was half strength ARN (Arnaud et al.10) supplemented with 2 gL-1 activated charcoal and solidified with 9 gL-1 Difco Agar®. The pH of the medium was adjusted to 5.8 before autoclaving (121°C, 20 min).
Root induction and acclimatization of plants
After the elongation phase, stems with at least 2.5 cm in length were used for root induction. Traceability of the explant was maintained according to its origin (genotype and cytokinin treatment used along the shoot induction stage). Subsequently, the stems were grown in root inducing medium (RIM), which consisted of 1/3 strength ARN basal medium supplemented with three different auxin treatments: A) 50 µM 1-naphthalene acetic acid (NAA), B) 50 µM indole-3-butyric acid (IBA), and C) a mixture of 40 µM NAA µM + 10 µM IBA. All the different media were solidified with 9 gL-1 Difco Agar®. The culture medium’s pH was adjusted to 5.8 before autoclaving (121°C, 20 min).
The stems were placed in the dark at 21 ± 1°C for 8 days. After this period, the stems were cultured in the root expression medium (REM), which consisted of 1/3 strength ARN basal medium (Arnaud et al.10) without plant growth regulators and solidified with 9 gL-1 Difco agar®. The pH of the medium was adjusted to 5.8 before autoclaving (121°C, 20 min). The cultures were placed in the growth chamber at a temperature of 21 ± 1°C, under 16-h photoperiod at 120 µmol m-2s-1. After six weeks of culture in REM medium, explants with visible roots were transferred to wet peat: vermiculite mixture (2:1, v/v) and acclimatized in the greenhouse under controlled conditions at 21 ± 1°C and decreasing humidity progressively along one month from 95 to 80%.
Data collection and statistical analysis
To assess the genotype’s effect on each of the variables of this study, an analysis of variance was performed (ANOVA), followed by a Tukey’s post hoc test (α=0.05). The genotype had a significant effect on all variables studied. For this reason and to obtain robust conclusions, the genotype factor was introduced into all the models as a block variable to reduce variability and analyze the effect of the other variable factors (the type of explant, cytokinin treatment, and auxin treatment) more accurately.
The shoot induction and the number of shoots per explant were recorded after six weeks. The experiment used a completely randomized design with 25 explants per treatment. Logistic regression was performed to assess the effect of the type of explant and the cytokinin treatment on the shoot induction percentage, followed by a Tukey’s post hoc test (α=0.05) for multiple comparisons. The number of shoots per explant variable did not fulfill homoscedasticity and normality assumptions for ANOVA; then, a Kruskal-Wallis was performed.
The root induction percentage, the number of roots per explant, and the root length were recorded after six weeks in REM. A completely randomized design was carried out using ten stems per cytokinin treatment. As an exception, in genotype 7, four stems were employed when K was used in the induction stage.
A logistic regression model was used to analyze the auxin and cytokinin treatment’s effect on the root induction percentage. Tukey’s post hoc test (α=0.05) was used for multiple comparisons. The number of roots per explant variable did not fulfill homocedasticity and normality assumptions for ANOVA; then, a Kruskal-Wallis was performed.
Data on the length of the longest root were square-root transformed, and an ANOVA was performed; multiple comparisons were made using Tukey’s post hoc test (α=0.05)
After twelve weeks under ex vitro conditions, the survival percentage was calculated. The survival percentage variable did not fulfill homocedasticity and normality assumptions for ANOVA; then a Kruskal-Wallis was performed.
RESULTS
Shoot induction
When the genotype effect on the shoot induction percentage was evaluated, genotypes 12 and 11 showed significantly higher percentages (98.98%)
than genotype 7 (79%, Table 1). As shown in Table 1, the number of shoots obtained in genotype 12 (5.04) was significantly higher than those obtained in genotypes 11 and 7
(3.21 and 3.28, respectively).
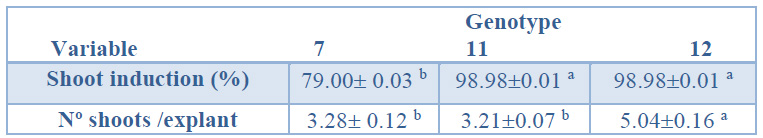
Data are presented as mean values ± S.E. Different letters within a row indicate significant differences (p<0.05).
Table 1. Shoot induction (%) and number shoots per explant from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al.10).
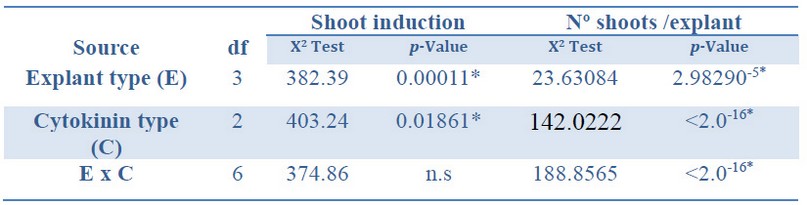
The deviance analysis of the factors studied showed a significant effect on both the shoot induction and the number of shoots per explant developed from each explant and cytokinin type.
There was a significant interaction between the explant type and the cytokinin type (Table 2).
*Significant differences at p<0.05, n.s Non-significant at p<0.05, df Degrees of freedom.
Table 2. Analysis of deviance for shoot induction (%) and number shoots per explant from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al.10).
Significantly higher shoot induction percentages were obtained in AP, BG and BP explants (between 91% and 94%) than in AG explants (84%) (Table 3).
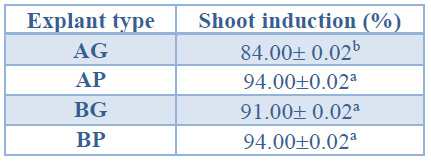
Data are presented as mean values ± S.E. Different letters indicate significant differences (p<0.05).
Table 3. Shoot induction (%) in different explant types from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al10) (apical explants of 1.5 cm in height (AG), apical explants of 1.0 cm height, C) basal explants of 1.5 cm height (BG) and basal explants of 1.0 cm in height (BP).
When considering the effect of the cytokinin type on shoot induction percentages, shoots induced with m-T showed significantly higher induction percentages than shoots induced with K. Shoots induced in the presence of BAP displayed intermediate values (92%) (Table 4).
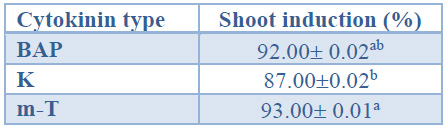
Data are presented as mean values ± S.E. Different letters indicate significant differences (p<0.05).
Table 4. Shoot induction (%) in different cytokinin type from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al10).
When evaluating the number of shoots per explant, a significant interaction between explant type and cytokinin was observed. The best results were obtained in AG, AP and BG explants induced with m-T and in AG explants induced with BAP (Figure 1). When BAP or m-T were used for induction, the worst results were obtained in BP explants. The lowest response was observed in treatment K, independently of the explant type.
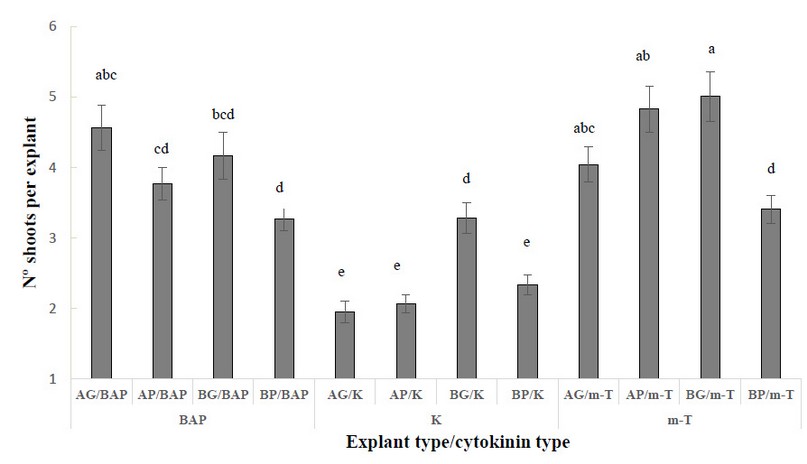
Figure 1. Number of shoots per explant in different explant types from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al. 10) supplemented with different cytokinins (BAP, m-T, K), (apical explants of 1.5 cm in length (AG), apical explants of 1.0 cm length (AP), basal explants of 1.5 cm length (BG) and basal explants of 1.0 cm in length (BP)). Data are presented as mean values ± S.E. Different letters indicate significant differences (p<0.05).
Despite there were no statistically significant differences between BG/m-T, AP/m-T, and AG/BAP, it is essential to mention that the appearance of induced shoots was different [Figure 2 (A-C)]. The shoots induced in BAP and K were greener and more vigorous (Figure 2 A) than the explants cultured in m-T (2 B, C). These explants from m-T treatments were more yellowish and less vigorous (Figure 2 B and C). Therefore, AG explants cultured in the presence of BAP showed the best results for shoot induction.

Figure 2. Shoot induction from Sequoia sempervirens elite adult trees cultured in ARN multiplication medium (Arnaud et al10) cultured for 6 weeks: (A) apical explant > 1.5 cm of length (AG) of genotype 7 on ARN medium + 4.4 µM BAP, bar = 3 mm ; (B) basal explant > 1.5 cm of length (BG) of genotype 7 on ARN medium + 4.4 µM m-T, bar = 5 mm; (C) apical explant < 1.5 cm of length (AP) explant of genotype 7 on ARN medium + 4.4 µM m-T, bar = 3 mm.
Since AG explants showed the best appearance, a comparative figure was done to describe the aspect of regenerated shoots in cytokinins’ presence (Figure 3). The most vigorous shoots were observed in the presence of BAP and K (Figure 3 (A-C, G-H).

Figure 3. Shoot induction from Sequoia sempervirens elite adult trees cultured in ARN multiplication medium (Arnaud et al10) cultured for 6 weeks: (A) apical explant > 1.5 cm of length (AG) of genotype 7 on ARN medium + 4.4 µM BAP, bar = 3 mm; (B) AG of genotype 11 on ARN medium + 4.4 µM BAP, bar = 3 mm; (C) AG explant of genotype 12 on ARN medium + 4.4 µM BAP, bar = 3 mm; (D) AG explant of genotype 7 on ARN medium + 4.4 µM m-T, bar = 3 mm; (E) AG explant > 1.5 of genotype 11 on ARN + 4.4 µM m-T, bar = 5 mm; (F) AG explant of genotype 12 on ARN medium + 4.4 µM m-T, bar = 5 mm; (G) AG of genotype 7 on ARN medium + 4.4 µM K, bar = 3 mm; (H) AG explant of genotype 11 on ARN medium +4.4 µM K, bar = 5 mm; (I) AG of genotype 12 on ARN medium + 4.4 µM KIN, bar = 3 mm.
Root induction
A significantly higher root induction percentage was obtained in genotype 12 (93%) (Table 5). The lowest response was genotype 7 (31%), and intermediate values were recorded for genotype 11 (55%). Also, a significantly higher number of roots per explant was observed in genotype 12 (4.73) than genotype 7 and 11 (Table 5).
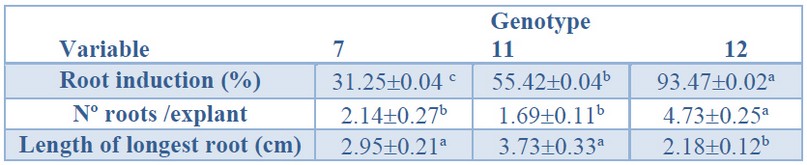
Data are presented as mean values ± S.E. Different letters within a row indicate significant differences (p<0.05).
Table 5. Root induction (%), number roots per explant, and the longest root from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al. 10).
On the contrary, significantly longer primary roots were recorded for genotype 11 (3.73 cm, Figure 4A) and genotype 7 (2.95 cm, Figure 4B) than for genotype 12 (2.18 cm, Figure 4C).

Figure 4. Root induction from Sequoia sempervirens elite adult trees micro shoots cultivated in vitro for 6 weeks on root expression medium (REM) (Arnaud et al. 10): (A) explant of genotype 7 induced in root inducing medium (RIM) (Arnaud et al. 10) + 50 µM NAA (BAP applied in shoot induction stage ), bar = 2 cm (B) explant of genotype 11 induced in RIM medium + 50 µM NAA (BAP applied in shoot induction stage ), bar = 2 cm; (C) explant of genotype 12 induced in RIM medium + 50 µM NAA (BAP applied in shoot induction stage), bar = 2 cm.
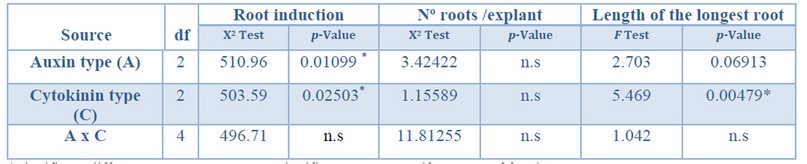
The auxin type and cytokinin type used in the multiplication stage showed a significant effect on the root induction percentage. Further, the cytokinin type showed a significant effect on the
longest root's length (Table 6). Regarding
the number of roots per explant, no significant differences were found for the variables tested.
*Significant differences at p<0.05, n.s Non-significant at p<0.05, df Degrees of freedom.
Table 6. Analysis of deviance for root induction (%), number roots per explant, and length of longest root from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al. 10).
As shown in Table 7, a significantly higher root induction percentage was obtained in the treatment with NAA (70%) than in treatments with IBA or IBA/NAA mixture. As mentioned before, there was no effect of the auxin treatment on the number of roots per explant (ranging from 2.98 to 3.94) or on the length of the primary root (ranging from 2.23 to 3.09 cm) (Table 7).
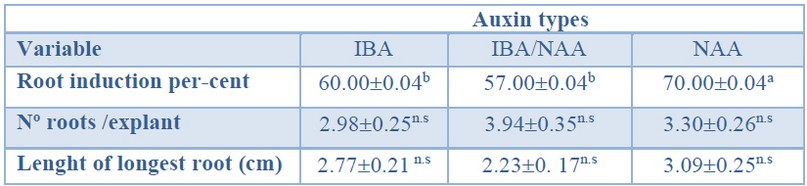
Data are presented as mean values ± S.E. Different letters within a row indicate significant differences (p<0.05).n.s Non-significant.
Table 7. Root induction (%), number of roots per explant and length of the longest root in Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al10) supplemented with different auxin types (IBA, IBA/NAA or NAA).
When evaluating the effect of the cytokinin type over root induction percentage, significantly higher values were obtained from shoots induced in BAP treatment when compared with those induced in m-T and K treatment (Table 8).
As aforementioned, no significant differences were found for the number of roots per explant, independently of the shoot induction treatment applied (ranging from 3.11 to 3.89) (Table 8).
The longest primary roots (3.32 cm) showed significantly higher values when shoots were induced in BAP treatment (Table 8).
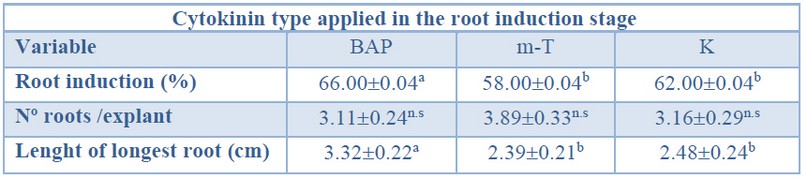
Data are presented as mean values ± S.E. Different letters within a row indicate significant differences (p<0.05).n.s Non-significant.
Table 8. Root induction (%), number roots per explant, and length of longest root in different cytokinin types from Sequoia sempervirens elite adult trees cultured in ARN medium (Arnaud et al10).
Acclimatization of rooted microplants
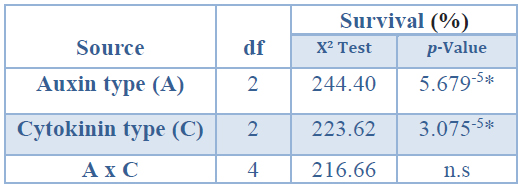
The analysis of deviance for survival (%) of rooted shoots propagated in vitro showed a significant effect of the auxin type applied in the root induction stage and of the cytokinin type
applied in the shoot induction phase (Table 9).
*Significant differences at p<0.05, n.s Non-significant at p<0.05, df Degrees of freedom.
Table 9. Analysis of deviance for survival (%) of rooted shoots propagated in vitro coming from Sequoia sempervirens elite adult trees, after twelve weeks under ex vitro conditions.
When the effect of auxin type on the survival percentage of rooted shoots was analyzed, the presence of IBA in the rooting medium provoked a significantly higher survival percentage (94%) than the mixture IBA/NAA and NAA treatments (Table 10).

Data are presented as mean values ± S.E. Different letters indicate significant differences (p<0.05).
Table 10. Ex Vitro survival (%) of in vitro rooted shoots from Sequoia sempervirens elite adult trees, shoots were rooted in ARN medium (Arnaud et al. 10) supplemented with different auxins (IBA, IBA/NAA, or NAA).
Regarding the effect of the cytokinin applied in the shoot induction stage as shown in Table 11, the shoots induced in K treatment showed a significantly higher ex vitro survival percentage than those induced in BAP or m-T treatments.

Data are presented as mean values ± S.E. Different letters indicate significant differences by Tukey’s post hoc test (p<0.05).
Table 11. Ex vitro survival (%) of in vitro rooted shoots from Sequoia sempervirens elite adult trees; shoots were propagated in ARN medium (Arnaud et al10) supplemented with different cytokinins (BAP, m-T or K).
At the end of the experiment, the micro propagated plants from the three elite adult trees evaluated showed normal development, profitable growth, and uniformity after 5 months under ex vitro conditions in the greenhouse (Figure 5).

Figure 5. Plants of Sequoia sempervirens cultured in vitro after 5 months growing in ex vitro conditions in the greenhouse, bar = 4 cm.
DISCUSSION
The genotype is an endogenous factor that has a significant role in regenerative potential in the overall repeatability and reliability of tissue culture protocol13. In this study, the shoot induction, the number of shoots per explant, the root induction, and the number of roots per explant were affected by the explant’s genotype. Our results are in concordance with the results reported by Meneguzzi et al. 2, who observed different genotypes’ responses in shoot multiplication of S. sempervirens. Similarly, Sul and Korban14 found differences in shoot proliferation and shoot elongation of S. sempervirens.
The organogenesis processes are influenced by endogenous and exogenous factors15,16,14. There are different exogenous factors as the components in the tissue culture medium or the culture’s environmental conditions. In this work, we observed that the type of explant and chemical conditions in the culture medium (cytokinin and auxin type) significantly affected shoot induction and rooting response.
During the evaluation of the shoot induction rate, the explants with the most extended length (AG, AP and BG) showed the best induction rate, and the highest number of shoots per explant were obtained. Similar results were found by Clapa et al. 17 and Meneguzzi18, who developed an in vitro protocol for S. sempervirens using shoot explants bigger than 1.5 cm in length. George16 reported that larger explants coming from more extensive parts of shoot apex or stem segments bearing one or more lateral buds could show advantages over smaller size explants.
Several studies about tissue culture protocols have reported positive effects on shoot proliferation, shoot multiplication rate, alleviating physiological disorders, better rooting, and acclimatization when using topolins for shoot induction 19,15. In our study, the explants induced in the presence of m-T exhibited the best results. In this sense, De Diego et al. 20, obtained a high rate of organogenic response in adult buds of Pinus sylvestris using m-T and suggested that it could be used as an alternative cytokinin BAP in micropropagation. Likewise, Bairu et al.21 obtained outstanding multiplication rates in Aloe polyphylla when used the same cytokinin. However, in the present study, these explants from m-T treatments were more yellowish and less vigorous. In contrast, in Prunus rootstocks, Pterocarpus marsupium, and Corylus colurna a positive effect of m-T on the growth and quality of micro propagated shoots was found 22,23,24.
According to Sul and Korban14, Meneguzzi et al. 18, Valverde et al. 25 and Montalbán et al. 26 in forestry species, BAP stimulated the axillary bud breakage and shoot elongation. In our study, a similar result was obtained in the BAP treatment. It is essential to mention that in the treatment with BAP the quality of the shoots formed (robustness and color) was superior to those shoots generated in the presence of m-T. In conclusion, in our study, BAP was the best cytokinin in shoot induction and the number of shoots per explant. This is in accordance with the results reported by Moncaleán et al. 27, Reanu et al. 16, Aremu et al. 15, and Bairu et al.21, confirming that BAP is the most used cytokinin in micropropagation due to its effectiveness and affordability. Regarding K treatment, in this work, we obtained the worst results in agreement with those found in Barleria greenii and Eucalyptus globulus28,29.
In this research, the NAA treatment showed the best result in root induction response. This agrees with the results reported in Sequoia sempervirens, Pinus radiata, Pinus pinaster, and other coniferous species 30,31,32,33,34. It is essential to mention that the rooting percentages obtained in the present studies were higher than those recorded by Huang et al. 35, who obtained about a 30% of rooting competence using adult stem sections of Sequoia sempervirens in the presence of IAA/K. The number of roots per explant obtained in the IBA/NAA treatment presented the highest values. In species such as Eucalyptus sideroxylon, Rosa hybrida and Citrus aurantiflolia the best root induction was obtained when using a mixture of IBA/NAA. Nonetheless, this treatment did not lead to the highest values in the number of roots per explant36,37,38. The highest value in the longest root length was also obtained in NAA treatment, being under the results obtained in Citrus aurantifolia38.
In our research, the induction stage’s cytokinin type had a significant effect on root induction. Bairu et al. 21, Werbrouck et al. 39, and Aremu et al. 15 found that m-T stimulated in vitro rooting activity, but the m-T treatment presented the worst in our work results. In this sense, Bairu et al. 40 and Escalona et al. 41 obtained negative carryover effects on rooting at too high m-T levels, so the m-T concentration applied in our cultures might have had a detrimental effect in the subsequent stages of development.
Regarding the acclimatization stage, the highest survival percentage was observed in plantlets rooted in IBA and developed in the presence of K. This result is in agreement with those found in Eucalyptus globulus, where the low concentration of IBA showed the best survival rates. In Arachis paraguariensis cultured in polyethylene terephthalate glycol vessel where IBA treatment was the best in survival42,43.
Aremu et al. 15 explained that cytokinins generally have inhibitory effects on rooting, resulting in low acclimatization rates afterward. In this sense, the plants from m-T treatment showed the lowest survival. In contrast, several studies have shown that plantlets coming from m-T induction treatments have been successfully acclimatized 44,21.
In conclusion, the role of auxins and cytokinins in the micropropagation of different types of explants and their relationship with the survival and acclimatization of seedlings in ex vitro conditions was analyzed. Our study results demonstrated that the apical explant ³ 1.5 cm length, and BAP showed the best results in the shoot induction stage. Moreover, in vitro shoots rooted with IBA, led toa higher ex vitro survival. Finally, the results shown allow the development of forthcoming studies for large-scale propagation of this species in semi-solid systems and bioreactors.
REFERENCES.
1. Mihaljević, S., Katavić, V. & Jelaska, S. Root formation in micropropagated shoots of Sequoia sempervirens using Agrobacterium. Plant Sci. 141, 73–80 (1999). . https://doi.org/10.1073/pnas.85.15.5536
2. Meneguzzi, A. et al. Shoot multiplication of two Sequoia sempervirens genotypes with addition of small concentrations of Kinetin. Pesqui. Florest. Bras. 39, 1-8 (2019). http://dx.doi.org/10.4336/2019.pfb.39e201701550
3. Taha, L. S., Taie, H. A. A., Metwally, S. A. & Fathy, H. M. Effect of laser radiation treatments on in vitro growth behavior, antioxidant activity and chemical constituents of Sequoia sempervirens. Res. J. Pharm. Biol. Chem. Sci. 5, 1024–1034 (2014).4. Fernando Otazua. Especies forestales 02.SECOYA, SEQUOIA, SEQUOIA ROJA. SEQUOIA DE CALIFORNIA (Sequoia sempervirens ENDL.) . Rev. la Asoc. For. Navarra 39, 06–09 (2016). https://www.foresna.org/wp-content/uploads/navarra-forestal39.pdf
5. Burns, Russell M., and Barbara H. Honkala, tech. coords. Silvics of North America: 1. Conifers; 2. Hardwoods. Agriculture Handbook 654.U.S. Department of Agriculture, Forest Service, Washington, DC, vol.2, 877 p. (1990). http:na.fs.fed.us/spfo/pubs/silvics_manual/Volume_1/vol11_Table_of_contents.html
6. Ozudogru, E. A. et al. Medium-term conservation of redwood (Sequoia sempervirens (D. Don.) Endl.) in vitro shoot cultures and encapsulated buds. Sci. Hortic. (Amsterdam). 127, 431–435 (2011). https://doi.org/10.1016/j.scienta.2010.10.013
7. Vengadesan, G., Ganapathi, A., Amutha, S. & Selvaraj, N. In vitro propagation of Acacia species – A review. Plant Sci. 163, 663–671 (2002). https://doi.org/10.1016/S0168-9452(02)00144-9
8. De Souza, R. A., Padilha Dantas, P. V., Cavalcante, P. D. F., Tenório, R. R. & Houllou, L. M. Basic procedure for the in vitro propagation of Brazilian trees for reforestation purposes. J. Environ. Anal. Prog. 2, 107–114 (2017). http://dx.doi.org/10.24221/jeap.2.2.2017.1139.107-114
9. Bonga, J. M. Conifer clonal propagation in tree improvement programs. In: Vegetative Propagation of Forest Trees. Yill-Sung Park, Jan M Bonga, Heung-Kyu Moon (eds). National Institute of Forest Science (NIFoS). Seoul, Korea. pp 3-31. (2016). https://www.researchgate.net/publication/299263644_Conifer_clonal_propagation_in_tree_improvement_programs
10. Arnaud, Y., Franclet, A., Tranvan, H. & Jacques, M. Micropropagation and rejuvenation of Sequoia sempervirens (Lamb) Endl: a review. Ann. des Sci. For. 50, 273–295 (1993). https://hal.archives-ouvertes.fr/hal-00882846
11. Sul, I. W. & Korban, S. S. Effect of different cytokinins on axillary shoot proliferation and elongation of several genotypes of Sequoia sempervirens. Vitr. Cell. Dev. Biol. – Plant 30, 131–135 (1994). https://doi.org/10.1007/BF02632201
12. Liu, C., Xia, X., Yin, W., Huang, L. & Zhou, J. Shoot regeneration and somatic embryogenesis from needles of redwood (Sequoia sempervirens (D.Don.) Endl.). Plant Cell Rep. 25, 621–628 (2006). https://doi.org/10.1007/s00299-006-0120-y
13. Coleman, G and Ernst, S. In vitro shoot regeneration of Populus deltoides: effect of cytokinin and genotype. Plant Cell Rep. 8, 459-462. https://doi.org/10.1007/BF00269048
14. Sul, I. W. & Korban, S. S. Direct shoot organogenesis from needles of three genotypes of Sequoia sempervirens. Plant Cell. Tissue Organ Cult. 80, 353–358 (2005). https://doi.org/10.1007/s11240-004-1365-1
15. Aremu, A. O., Bairu, M. W., Doležal, K., Finnie, J. F. & Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell. Tissue Organ Cult. 108, 1–16 (2012). https://doi.org/10.1007/s11240-011-0007-7
16. George, E. F., Hall, M. a., & Klerk, G.-J. De. Micropropagation: Uses and Methods. Plant Propagation by Tissue Cutlure 3rd Edition Vol 1. The Background. Book. Springer Berlin Heidelberg (2008). https://doi.org/10.1007/978-1-4020-5005-3_1
17. Clapa, D. et al. Sequoia sempervirens in Transylvania. Acta Hortic. 885, 85–90 (2010). https://doi.org/10.17660/ActaHortic.2010.885.11
18. Meneguzzi, A. et al. Agar and activated charcoal influence the in vitro development of shoot and root Sequoia. Rev. Ciencias Agroveterinarias 18, 20–24 (2020). https://doi.org/10.5965/2238117118e2019020
19. Moyo, M. et al. Deciphering the growth pattern and phytohormonal content in Saskatoon berry (Amelanchier alnifolia) in response to in vitro cytokinin application. N. Biotechnol. 42, 85–94 (2018). https://doi.org/10.1016/j.nbt.2018.02.001
20. De Diego, N., Montalbán, I. A. & Moncaleán, P. In vitro regeneration of adult Pinus sylvestris L. trees. South African J. Bot. 76, 158–162 (2010). https://doi.org/10.1016/j.sajb.2009.09.007
21. Bairu, M. W., Stirk, W. A., Dolezal, K. & Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell. Tissue Organ Cult. 90, 15–23 (2007). https://doi.org/10.1007/s11240-007-9233-4
22. Gentile, A. et al. Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell. Tissue Organ Cult. 118, 373–381 (2014). https://doi.org/10.1007/s11240-014-0489-1
23. Ahmad, A. & Anis, M. Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium Roxb.: a potential drug-yielding tree. J. Plant Growth Regul. 38, 1007–1016 (2019). https://doi.org/10.1007/s00344-018-09910-9
24. Gentile, A., Frattarelli, A., Nota, P., Condello, E. & Caboni, E. The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell. Tissue Organ Cult. 128, 693–703 (2017). https://doi.org/10.1007/s11240-016-1150-y
25. Valverde-Cerdas, L., Alvarado, L. & Hine, A. Micropropagation of clones from controlled crosses of Gmelina arborea in Costa Rica. New For. 28, 187–194 (2004). https://doi.org/10.1023/B:NEFO.0000040945.75019.38
26. Montalbán, I. A., De Diego, N. & Moncaleán, P. Testing novel cytokinins for improved in vitro adventitious shoots formation and subsequent ex vitro performance in Pinus radiata. Forestry 84, 363–373 (2011). https://doi.org/10.1093/forestry/cpr022
27. Moncaleán, P. et al. Organogenic responses of Pinus pinea cotyledons to hormonal treatments: BA metabolism and cytokinin content. Tree Physiol. 25, 1–9 (2005). https://doi.org/10.1093/treephys/25.1.1
28. Amoo, S. O., Finnie, J. F. & van Staden, J. The role of meta-topolins in alleviating micropropagation problems. Plant Growth Regul. 63, 197–206 (2011). https://doi.org/10.1007/s10725-010-9504-7
29. Bennett, I.J., McComb, J.A., Tonkin, C.M., McDavid, D.A.J. Alternating cytokinins in multiplication media stimulates in vitro shoot growth and rooting of Eucalyptus globulus Labill. Annals of Botany,74, 53-58 (1994).
https://doi.org/10.1006/anbo.1994.1093.
30. Halmagyi, A. et al. Effects of naphthenic acids on rooting of in vitro grown Sequoia sempervirens. Acta Hortic. 885, 139–144 (2010). https://doi.org/10.17660/ActaHortic.2010.885.18
31. Montalbán, I. A. & Moncaleán, P. Rooting of Pinus radiata somatic embryos: factors involved in the success of the process. J. For. Res. 30, 65–71 (2019). https://doi.org/10.1007/s11676-018-0618-5
32. De Diego, N., Montalbán, I. A., Fernandez De Larrinoa, E. & Moncaleán, P. In vitro regeneration of Pinus pinaster adult trees. Can. J. For. Res. 38, 2607–2615 (2008). https://doi.org/10.1139/X08-102
33. De Diego, N., Montalbán, I. A. & Paloma, M. Improved micropropagation protocol for maritime pine using zygotic embryos. Scand. J. For. Res. 26, 202–211 (2011). https://doi.org/10.1080/02827581.2011.559174
34. Ragonezi, C. et al. Adventitious rooting of conifers: Influence of physical and chemical factors. Trees – Struct. Funct. 24, 975–992 (2010). https://doi.org/10.1007/s00468-010-0488-8
35. Huang, L. C. et al. Rejuvenation of Sequoia sempervirens by repeated grafting of shoot tips onto juvenile rootstocks in vitro: Model for phase reversal of trees. Plant Physiol. 98, 166–173 (1992). https://doi.org/10.1104/pp.98.1.166
36. Cheng, B., Peterson, C. M. & Mitchell, R. J. The role of sucrose, auxin and explant source on in vitro rooting of seedling explants of Eucalyptus sideroxylon. Plant Sci. 87, 207–214 (1992). https://doi.org/10.1016/0168-9452(92)90152-C
37. Khosh- Khui and Sink Scientific. Rooting enhancemet of Rosa hybrida for tissue culture propagation. Sci. Hortic. 17, 371–376 (1982). https://doi.org/10.1016/0304-4238(82)90118-2.
38. Al-Bahrany, A. M. Effect of phytohormones on in vitro shoot multiplication and rooting of lime Citrus aurantifolia (Christm.) Swing. Sci. Hortic. (Amsterdam). 95, 285–295 (2002). https://doi.org/10.1016/S0304-4238(01)00349-1
39. Werbrouck, S., Strnad, M.,Van Onckelen, H. Meta‐topolin, an alternative to benzyladenine in tissue culture? Physiologia Plantarum. 98, 291–297 (1996). https://doi.org/10.1034/j.1399-3054.1996.980210.x
40. Bairu, M. W., Stirk, W. A., Doležal, K. & Van Staden, J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ (Musa spp. AAA). Plant Cell. Tissue Organ Cult. 95, 373–379 (2008). https://doi.org/10.1007/s11240-008-9451-4
41. Escalona, M.;Cejas, I.; Gonzáles-Olmedo, J.; Capote, I.; Roels, S.;Cañal, M.J.;Rodriguez, R.; Sandoval Fernandez, J.A.; Debergh, P.The effect of meta-topolin on plantain propagation using a temporary immersion bioreactor. Infomusa 12, 28-30 (2003). https://www.musalit.org/seeMore.php?id=8153
42. Bennett, I.J; McDavid, D.A.j; McComb, J. The influence of ammonium nitrate, pH and indole butyric acid on root induction and survival in soil of micropropagated Eucalyptus globulus. Biol. Plant. 47, 355–360 (2003). https://doi.org/10.1023/B:BIOP.0000023877.21262.a5
43. Aina, O. O., Quesenberry, K. H. & Gallo, M. Culture vessel and auxin treatments affect in vitro rooting and ex vitro survival of six Arachis paraguariensis genotypes. Sci. Hortic. (Amsterdam). 183, 167–171 (2015). http://dx.doi.org/10.1016/j.scienta.2014.12.006
44. Naaz, A., Hussain, S. A., Anis, M. & Alatar, A. A. Meta-topolin improved micropropagation in Syzygium cumini and acclimatization to ex vitro conditions. Biol. Plant. 63, 174–182 (2019). https://doi.org/10.32615/bp.2019.020
Received: 18 December 2020
Accepted: 10 January 2021
1,2Alejandra Rojas Vargas, Castander-Olarieta A.2, Moncaleán P.2, Montalbán I.A.2*
1Instituto de Investigación y Servicios Forestales, Universidad Nacional, Heredia, Costa Rica.
2 NEIKER-BRTA, Department of Forestry Sciences, Arkaute.01192, Spain.
*Corresponding author. imontalban@neiker.eus.