Vol 5 No 4 2020 – 5
INVESTIGATION / RESEARCH
Treatment of SARS-CoV-2 (COVID-19) cases by the oral administration of montelukast tablets
Ameneh Norouzi1*
Available from: http://dx.doi.org/10.21931/RB/2020.05.04.5
ABSTRACT
According to the hypothesis, montelukast may have therapeutic action against severe acute respiratory syndrome (SARS) occurred by coronavirus 2 (CoV-19). The research was aimed to evaluate the therapeutic effects of montelukast tablet on coronavirus infectious disease (COVID-19) patients. A total of 20 COVID-19 confirmed patients were included in this study. The presence of COVID-19 infections in all patients was confirmed using real-time polymerase chain reaction (PCR) and computerized tomography (CT) scan. Confirmed cases were treated with oral administration of montelukast (10 mg) tablet for 10 days. The study population was included 18 to 82 years old patients (10 males and 10 females). The mean age of studied men and women individuals were 44.7±17 and 41±17.45 years, respectively. Frequency of respiratory distress, cough, abdominal cramps/diarrhea, fever, and odor disorder clinical signs amongst the examined patients were 85%, 90%, 20%, 70%, and 65%, respectively. Our findings revealed that all patients who were received 10 days of oral administration of montelukast tablets (10 mg) were recovered from the COVID-19 disease.
Additionally, all of the clinical signs of COVID-19 patients, including respiratory distress, cough, and odor disorder, were gradually disappeared. Our findings revealed that widespread oral administration of montelukast tablets (10 mg) is a potential treatment for COVID-19 disease. However, several double-blind and multifactorial clinical trials should perform to determine the other clinical aspects of the treatment of COVID-19 patients by oral administration of montelukast.
Keywords: SARS-COVID-19, Treatment, Montelukast.
INTRODUCTION
Infectious diseases remain a threatening issue for human health despite the high development of medical sciences 1,18,30,35,36. Severe Acute Respiratory Syndrome (SARS)-Corona Virus Diseases-19 (CoVID-19) (SARS-CoVID-19) which also known as SARS-CoV-2, is the essential threatening disease in 2019 and 2020 years all around the world 57.
Coronaviruses (CoVs) belong to the family of Coronaviridae, the order Nidovirales, and the genus Coronavirus, with a positive sense, single-stranded RNA genome. Human coronaviruses (HCoVs), are documented as respiratory pathogens related to respiratory and intestinal infections with various severities from the usual cold to pneumonia and bronchiolitis to death. COVID-19, as a deadly disease is occurred owing to the activity of SARS-CoV-2, accounted as a global public health concern 31,43.
Rendering the World Health Organization’s (WHO)’ report, the epidemic of COVID-19 so far registered 26,763,217 cases and 876,616 deaths worldwide (9) WHO showed that the highest numbers of COVID-19 new cases had been reported from Iraq, Iran, Morocco, Saudi Arabia, Kuwait 34. In Iran, several cumulative cases and also cumulative death of Covid-19 were 384,666 and 22,154, respectively 34.
Despite the worldwide spread, there were no definitive treatments for the COVID-19 38. Thus, there was a large demand to introduce a novel therapeutic option for the treatment of the cases of COVID-19.
It has been theorized that Montelukast, an antagonist of the cysteinyl leukotriene (cysLT) receptor with potential antioxidant and anti-inflammatory effects, may reduce the development of the COVID-19 infection 16. Montelukast has an effect on events developing with Angiotensin-converting enzyme (ACE) receptors, and also has an anti-inflammatory effect with bradykinin and leukotriene antagonism; Because of COVID-19 has entry into the cell through ACE receptors and caused mortality due to excessive inflammatory processes, it was thought that montelukast might have a therapeutic effect on the progression of COVID-19 infection 20.
According to the high importance of COVID-19, the absence of effective treatment, and the probable effect of the montelukast, the present research was done to assess the effects of oral administration of montelukast as a therapeutic agent for the treatment of SARS-CoV-2 patients.
MATERIALS AND METHODS
Ethics and consents
The present survey was conducted on volunteer patients who suffered from SARS-COVID-19 disease. Informed consent was obtained from the patients or their parents involved in this survey. Additionally, all the identity and personal information of the patients participating in this study remained secret. Ethical principles of patient care and sampling were also observed. Serious efforts were made to reduce patients’ pain and anxiety during the research process.
Study population
A total of 20 male and female ≥18 years old patients with clinical signs of COVID-19 disease were confirmed by the computerized tomography (CT)-scan and Real-Time Polymerase Chain Reaction (PCR) were included in this study.
Inclusion and exclusion criteria
All patients confirmed to be infected with the COVID-19 virus through the nucleic acid detection by the Real-Time PCR assay, and positive outcomes of chest CT-scan were included in the study. Additionally, clinical signs of the disease were considered. If both chest CT-scan and Real-Time PCR test showed negative findings of COVID-19 disease for any patients, they were excluded from the study. Additionally, patients who died during the study and also those who used from other therapeutic options against SARS-COVID-19 were excluded from the research. Furthermore, patients with progressive and autoimmune diseases were excluded from the study.
COVID-19 virus detection and diseases confirmation in the study population
Patients were confirmed by Reverse transcription-Real-Time PCR (RT-Real-Time PCR) using throat swab specimens from the upper respiratory tract or clinically diagnosed based on lung imaging features, specially Chest CT scan ground glass pathognomonic features consistent with coronavirus pneumonia, depending on the physician’s orders.
The presence of the COVID-19 virus was examined using the RT-Real-Time PCR method. For this purpose, the method described previously was used 47. Throat swab samples were used for RNA extraction. Lesser than 37 cycle threshold values (Ct-value) were considered as a positive, while those of higher than 40 were recognized as negative. Additionally, a chest CT scan was performed, and its results were analyzed according to lung involvements, density (ground‐glass and consolidations), and central to peripheral distribution 52.
Treatment protocol
Confirmed patients with CT-scan and RT-Real-Time PCR were subjected to oral administration of montelukast tablets (10 mg, Dr. Abidi Co, Tehran, Iran) for about 10 days. Patients were used two montelukast tablets on the first day of the experiment and then only one table from days 2 to 10 of the experiment. Routine cares of COVID-19 were addressed for all patients 24,44.
Statistical analysis
All data collected from the study were transferred to Microsoft Excel software (Microsoft Corp., Redmond, WA, USA). Statistical analyses were performed using SPSS 21.0 (Statistical Package for the Social Sciences) software (SPSS Inc., Chicago, IL, USA). Variables were defined as frequencies and percentages. A comparison of the differences was conducted using the Chi-square test. P-value<0.05 was considered statistically significant.
RESULTS
COVID-19 identification
The present survey was performed to assess the therapeutic effects of oral administration of montelukast tablet (10 mg) in patients suffered from COVID-19 disease. Figure 1 represents the pattern of lung CT-scan in some of the examined COVID-19 patients. As shown, multi-lobar and bilateral ground‐glass opacities are seen in both lungs, mostly in mid to lower lungs, although all lobes are affected, with a peripheral subpleural distribution. Additionally, the presence of the COVID-19 virus was identified by the Real-Time PCR method in all examined patients.

Figure 1. The pattern of lung CT-scan in some of the examined COVID-19 patients.
Study population
Table 1 represents the characters of the study population of COVID-19 patients. The study population consisted of 18 to 82 years old male and female patients. The total distribution of male and female individuals amongst all 20 examined COVID-19 patients were 50% (10/20) and 50% (10/20), respectively. The mean age of male and female patients was 44.7±17 and 41±17.45 years, respectively. Total distribution of respiratory distress, cough, abdominal cramps/diarrhea, fever, and odor disorders amongst all examined patients were 85%, 90%, 20%, 70%, and 65%, respectively. However, there were no statistically significant differences between and within population-based data presented in this study (P <0.05). No deaths and also progressive and autoimmune diseases were found in the study population. Additionally, none of the patients were received any other anti-viral or other therapeutic options.
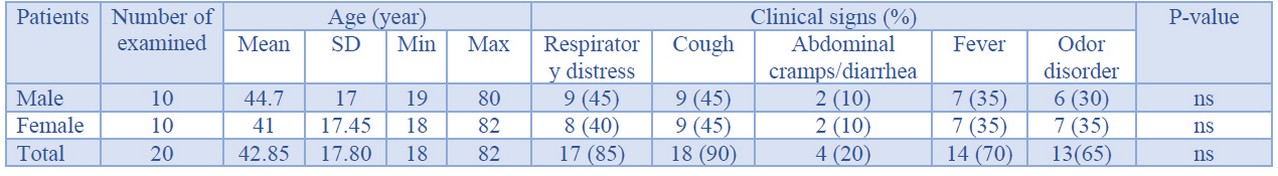
Table 1. Characters of the study population in the patients suffered from COVID-19.
Treatment outcomes
Our findings revealed that all of the COVID-19 patients who were treated with 10 days of oral administration of montelukast tablets (10 mg) were recovered from the COVID-19 disease. Findings revealed that all of the clinical signs of the COVID-19, including respiratory distress, cough, and odor disorder, were gradually disappeared. Respiratory distress and cough were the first clinical signs that disappeared after the montelukast administration. However, cramps and diarrhea continued for about a few days after the study experiment. Patients’ odor appeared one to two days after the end of the study experiment. There were no detectable side effects after 10 days of oral administration of montelukast tablets in examined patients.
DISCUSSION
From the first days of the occurrence of COVID-19 disease, diverse kinds of therapeutic options have been presented to patients 38. However, none of them weren’t introduced as definitive and affected treatment 46. Otherwise, no specific anti-viral option has been established as an effective agent against COVID-19 virus 26. Despite the significant anti-viral effects of remdesivir 50, teicoplanin 55, nelfinavir 53, colistin, valrubicin, icatibant, bepotastine, epirubicin, epoprostenol, vapreotide, aprepitant, caspofungin, and perphenazine 28, rupintrivir and lopinavir 42, ebselen 23, beclabuvir and saquinavir 41, indinavir, cobicistat, caspofungin acetate, and atazanavir 15, carfilzomib, eravacycline, valrubicin, elbasvir, and streptomycin 48, thymopentin, carfilzomib, and saquinavir 49, ledipasvir and velpatasvir 14, atazanavir, efavirenz, ritonavir, and dolutegravir 5, mycophenolic acid, grazoprevir, telaprevir, and boceprevir 19, formoterol and chloroquine 4, eriodictyol, isoniazid pyruvate, nitrofurantoin, cepharanthine, ergoloid, and hypericin 45, ikarugamycin and molsidomine 27, baricitinib 37, and lithium 33 drugs, none of them weren’t introduced as the definitive treatment of COVID-19 disease. Thus, it is essential to work on other therapeutic candidates for the treatment of lethal COVID-19 disease.
The present survey disclosed that the oral administration of montelukast tablets (10 mg) for about 10 days could decrease the risk of death in COVID-19 patients. Additionally, findings revealed that all of the clinical signs of COVID-19 patients were gradually disappeared after treatment. Thus, this is the report of the potential of treatment of COVID-19 patients by the oral administration of montelukast tablets (10 mg) for about 10 days.
Diverse researches have focused on the impact of montelukast as a probable candidate for the treatment of COVID-19 6,20. Montelukast is initially and typically used for the treatment of asthma, but it has some anti-viral activities, which have been assessed in some surveys but not approved well 17,21,22,25. Bozek et al. 9 surveyed elderly patients who suffered from asthma who were treated with montelukast. They found that montelukast administration provided benefits and improved asthma control, while other drugs did not lead to a comprehensive healing of asthma. Then, they performed a retrospective observational survey as an extension of the above project with the same patients on morbidity due to COVID-19 10. They found a significant reduction in SARS-CoV-2 infection in the group of elderly asthmatic patients treated with montelukast. Nowak, J.K. & Walkowiak, J. 33 reported that the administration of antiallergic medications such as montelukast and levocetirizine, and their combination could be effective against COVID-19. In keeping with this, the high safety of montelukast administration for the treatment of viral and respiratory diseases has been reported previously 2,7,11,12. Mansour, S. et al. 29 surveyed computational docking to assess the effects of montelukast on COVID-19 protease catalytic site. They showed a possible inhibitory portion of montelukast in binding to the Mpro catalytic site of the COVID-19 virus, which may modulate and inhibit the viral replication.
Reports indicated that ACE receptors are an essential target of the entrance of COVID-19 into the cell, which causes severe pneumonia and subsequently increases in the mortality rate 13,40. The cough that can progress by ACE inhibition is caused by increased bradykinin and its bronchoconstrictor result, and montelukast, an antagonist of leukotriene D4 (LTD4), has a repressive effect on hypersensitivity of the airway induced by bradykinin 8,32. Respiratory failures and acute respiratory distress syndrome (ARDS) are the most causes of death in the COVID-19 patients 39,56. ARDS is mainly caused severe lung injuries with a high inflammatory response with an increase in the levels of Interleukin-6 (IL-6), IL-8, and IL-1 and tumor necrosis factor (TNF) in an initial phase and other pro-inflammatory cytokines in the later phase of the disease 13. These procedures increased leukocyte migration to the affected region, which causes leukocytes accumulation, reactive oxygen secretion, and protease production with severe damages to capillary endothelium and alveolar epithelium 40. Surveys revealed that the application of montelukast caused a severe reduction in the levels of TNF-α, IL-6, and IL-1 32. Additionally, the inhibitory effect of montelukast toward tracheal contraction induced by bradykinin has also been established 8,13,32,40.
Furthermore, it has been established that the montelukast decreases the levels of oxidative stress in cells 39. Investigations described that high doses and intravenous (IV) administration of montelukast caused a significant decrease in the expression of IL-4, IL-5, IL-13 proteins in the lungs, and anti-inflammatory activities by the T-helper type-2 cytokines suppression 51,56. Moreover, montelukast administration caused significant decrease in the frequency and severity of wheezing in patients with an upper respiratory tract infection caused by coronavirus, adenovirus, metapneumovirus, and influenza 3,8,11. Thus, montelukast has an effect on events developing with ACE receptors, and also has an anti-inflammatory effect with bradykinin and leukotriene antagonism; Because of COVID-19 has entry into the cell through ACE receptors and caused mortality due to excessive inflammatory processes, it was thought that montelukast may have a limiting effect on the progression of the disease on COVID-19 infection. Anti-COVID-19 activities of montelukast were confirmed in the present survey.
According to our hypothesis, montelukast caused healing effects and prevented from cough, respiratory distress, congestion, and suffocation of patients suffered from COVID-19 and provided more time for the immune system to fight the virus.
The author of the present study guesses that the COVID-19 virus causes immunodeficiency reactions by disrupting the complement system, and lung damage is not the direct function of the virus 54. Thus, patients faced with immunodeficiency reactions caused severe damages to lung and consequent death. Proof of this hypothesis offers practical approaches to develop new drug therapies against COVID-19 disease. Otherwise, the COVID-19 virus is probably caused severe disorders in the immune system of patients and caused subsequent disturbances in the complement and cytokines systems, which resulted in the appearance of clinical signs and death in patients. This is the possible reason for the lack of anti-viral options used for the treatment of disease. However, the establishment of this hypothesis needs further researches.
Our observations are limited by the small group of patients and lack of some other supplementary tests, which may be partly due to avoid wasting time in the publication of the valuable results of this research to scientists around the world and saving millions of lives. Additionally, this is a preliminary work due to the size of the sample, the high dispersion in the age group, and the absence of a control group. However, the author potentially recommended oral administration of montelukast (10 mg) as a potential therapeutic option for the treatment of COVID-18 disease all-around the world.
CONCLUSION
According to the results of the present survey and also those of other review articles, montelukast is the potential treatment for COVID-19 disease. Our finding revealed that all of the COVID-19 patients were treated after 10 days of oral administration of montelukast tablets (10 mg) for about 10 days. Thus, the general oral administration of montelukast tablets (10 mg) is potentially suggested as a potential treatment for COVID-19 disease—this a preliminary study on the effect of montelukast as a potential treatment for COVID-19 infections. Thus, several double-blind and multifactorial clinical trials should perform to determine the other clinical aspects of the treatment of COVID-19 disease by oral administration of montelukast.
Acknowledgments
The author applied all coordination, work, and costs. There was no funding information and also no conflict of interest to the state.
REFERENCES
1. Abdolmaleki, Z., Mashak, Z., & Dehkordi, F.S. Phenotypic and genotypic characterization of antibiotic resistance in the methicillin-resistant Staphylococcus aureus strains isolated from hospital cockroaches. Antimicrob. Resist. Infect. Control. 8, Page (2019).
2. Ahmad, A., Waseem, T., Butt, N.F., Randhawa, F.A., Malik, U., & Shakoori, T.A. Montelukast reduces the risk of dengue shock syndrome in dengue patients. Trop. Biomed. 35, Page (2018).
3. Andra, I.S. et al. Assertion for montelukast in the covid-19 pandemics? Farmacia. 68, Page (2020).
4. Arya, R., Das, A., Prashar, V., & Kumar, M. Potential inhibitors against papain-like protease of novel coronavirus (SARS-CoV-2) from FDA approved drugs. (2020).
5. Beck, B.R., Shin, B., Choi, Y., Park, S., & Kang, K. Predicting commercially available anti-viral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Structural, Biotech. J. (2020).
6. Bhattacharyya, D. Reposition of montelukast either alone or in combination with levocetirizine against SARS-CoV-2. Med. Hypoth.144, Page (2020).
7. Bisgaard, H. et al. Study of montelukast for the treatment of respiratory symptoms of post–respiratory syncytial virus bronchiolitis in children. Am. J. Respir. Crit. Care. Med. 178, Page (2008).
8. Bisgaard, H., Flores-Nunez, A., Goh, A., Azimi, P., Halkas, A., & Malice, M.P. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am. J. Respir. Critical. Care. Med. 178, Page (2008).
9. Bozek, A., Warkocka-Szoltysek, B., Filipowska-Gronska, A., & Jarzab, J. Montelukast as an add-on therapy to inhaled corticosteroids in the treatment of severe asthma in elderly patients. J. Asthma. 49, Page (2012).
10. Bozek, A., & Winterstein, J. Montelukast’s ability to fight COVID-19 infection. J. Asthma. 2020, Page (2020).
11. Brodlie, M., Gupta, A., Rodriguez-Martinez, C.E., Castro-Rodriguez, J.A., Ducharme, F.M., & McKean, M.C. Leukotriene receptor antagonists as maintenance and intermittent therapy for episodic viral wheeze in children. Cochr. Database. Syst. Rev. (2015 ).
12. Bruce Chandler, Publication no. . Levocetirizine and montelukast for the treatment of influenza, common cold and inflammation, European. Patent. office. (17 Oct., 2018).
13. Chen, X., Zhang, X., & Pan, J. Medical science monitor: Effect of Montelukast on Bronchopulmonary Dysplasia (BPD) and Related Mechanisms. Int. Med. J. Exp. Clin. Res. 13, Page (2019 ).
14. Chen, Y.W., Yiu, C.-P., & Wong, K.-Y. Prediction of the 2019-nCoV 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. ChemRxiv. (2020).
15. Contini, A. Virtual screening of an FDA approved drugs database on two COVID-19 coronavirus proteins. (2020).
16. Copertino, D.C., Duarte, R.R., Powell, T.R., de Mulder Rougvie, M., & Nixon, D.F. Montelukast drug activity and potential against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). J. Med.Virol. (2020).
17. Crimi, N. et al. Inhibitory effect of a leukotriene receptor antagonist (montelukast) on neurokinin A-induced bronchoconstriction. J. Allergy. Clin. Immunol. 111, Page (2003).
18. Dehkordi, F.S., Gandomi, H., Basti, A.A., Misaghi, A., & Rahimi, E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob. Resist. Infect.Control. 6, Page (2017).
19. Elfiky, A., & Ibrahim, N.S. Anti-SARS and anti-HCV drugs repurposing against the Papain-like protease of the newly emerged coronavirus (2019-nCoV). (2020).
20. Fidan, C., & Aydoğdu, A. As a potential treatment of COVID-19: Montelukast. Med. Hypothes. 142, Page (2020).
21. Fitzgerald, D.A., & Mellis, C.M. Leukotriene receptor antagonists in virus-induced wheezing. Treatments in respiratory medicine. 5, Page (2006).
22. Han, J. et al. Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytial virus. American. J.Respirat. Critical. Med. 182, Page (2010).
23. Jin, Z. et al. Structure-based drug design, virtual screening and high-throughput screening rapidly identify anti-viral leads targeting COVID-19. BioRxiv. (2020).
24. Kang, C., Yang, S., Yuan, J., Xu, L., Zhao, X., & Yang, J. Patients with chronic illness urgently need integrated physical and psychological care during the COVID-19 outbreak. Asian. J. Psychiatry. 51, Page (2020).
25. Kloepfer, K.M. et al. Effects of montelukast on patients with asthma after experimental inoculation with human rhinovirus 16. Annals of Allergy, Asthma. Immunol. 106, Page (2011).
26. Kouznetsov, V.V. COVID-19 treatment: Much research and testing, but far, few magic bullets against SARS-CoV-2 coronavirus. European. J. Med. Chem. 203, Page (2020).
27. Li, Z., Bai, T., Yang, L., & Hou, X. Discovery of potential drugs for COVID-19 based on the connectivity map. (2020).
28. Liu, X., & Wang, X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Gen. Genom. 47, Page (2020).
29. Mansour, S. et al. A Case for Montelukast in COVID-19: “The use of Computational Docking to estimate the effects of Montelukast on potential viral main protease catalytic site. Molecular. Biol. Virol. (2020).
30. Mashak, Z., Jafariaskari, S., Alavi, I., Shahreza, M.S., & Dehkordi, F.S. Phenotypic and Genotypic Assessment of Antibiotic Resistance and Genotyping of vacA, cagA, iceA, oipA, cagE, and babA2 Alleles of Helicobacter pylori Bacteria Isolated from Raw Meat. Infect. Drug. Resist. 13, Page (2020).
31. Mirzaie, A., Halaji, M., Dehkordi, F.S., Ranjbar, R., & Noorbazargan, H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complementary. Therap. Clin. Practice. (2020).
32. Noor, A., Najmi, M.H., & Bukhtiar, S. Effect of Montelukast on bradykinin-induced contraction of isolated tracheal smooth muscle of guinea pig. Indian J Pharmacol. 43, Page (2011).
33. Nowak, J.K., & Walkowiak, J. Is lithium a potential treatment for the novel Wuhan (2019-nCoV) coronavirus? A scoping review. F1000Research. 9, Page (2020).
34. Organization, W.H. Coronavirus disease ( COVID-19): weekly epidemiological update. (2020).
35. Rahi, A., Kazemeini, H., Jafariaskari, S., Seif, A., Hosseini, S., & Dehkordi, F.S. Genotypic and Phenotypic-Based Assessment of Antibiotic Resistance and Profile of Staphylococcal Cassette Chromosome mec in the Methicillin-Resistant Staphylococcus aureus Recovered from Raw Milk. Infect. Drug. Resist. 13, Page (2020).
36. Ranjbar, R., Farsani, F.Y., & Dehkordi, F.S. Phenotypic analysis of antibiotic resistance and genotypic study of the vacA, cagA, iceA, oipA and babA genotypes of the Helicobacter pylori strains isolated from raw milk. Antimicrobial Resistance & Infection Control. 7, Page (2018).
37. Richardson, P. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England). 395, Page (2020).
38. Rismanbaf, A. Potential treatments for COVID-19; a narrative literature review. Arch. Academic. Emergency. Med. 8, Page (2020).
39. Rodriguez-Morales, A.J., Cardona-Ospina, J.A., Gutierrez-Ocampo, E., Villamizar-Pena, R., Holguin-Rivera, Y., & Escalera-Antezana, J.P. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel. Med. Infect. Dis. (2020).
40. Sarzi-Puttini, P., Giorgi, V., Sirotti, S., Marotto, D., Ardizzone, S., & Rizzardini, G. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 38, Page (2020).
41. Sekhar, T. Virtual Screening based prediction of potential drugs for COVID-19. Preprints. (2020).
42. Shang, J. et al. The treatment and outcomes of patients with COVID-19 in Hubei, China: a multi-centered, retrospective, observational study. (2020).
43. Sheikhshahrokh, A. et al. Frontier therapeutics and vaccine strategies for sars-cov-2 (COVID-19): A review. Iranian. J. Public. Health. 49, Page (2020).
44. Shih, H.-I., Wu, C.-J., Tu, Y.-F., & Chi, C.-Y. Fighting COVID-19: a quick review of diagnoses, therapies, and vaccines. Biomed. J. (2020).
45. Smith, M., & Smith, J.C. Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. (2020).
46. Tu, Y.-F. et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Molecul. Sci. 21, Page (2020).
47. Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 323, Page (2020).
48. Wang, J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inform. Model. (2020).
49. Wang, Q., Zhao, Y., Chen, X., & Hong, A. Virtual screening of approved clinic drugs with main protease (3CLpro) reveals potential inhibitory effects on SARS-CoV-2. J. Biomolecul. Dynamics. (2020).
50. Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 395, Page (2020).
51. Wu, A.Y., Chik, S.C., Chan, A.W., Li, Z., Tsang, K.W., & Li, W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin Exp Allergy. 33, Page (2003).
52. Xia, X. et al. Analyzing the Early CT findings and Clinical Features of 12 Patients with 2019 Novel Coronavirus Disease (COVID-19) in China. Iranian. Red. Crescent. Med. J. 22, Page (2020).
53. Xu, Z. et al. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. BioRxiv. (2020).
54. Yazdanpanah, F., Hamblin, M.R., & Rezaei, N. The immune system and COVID-19: Friend or foe? Life Science. 256:117900 (2020).
55. Zhang, J. et al. Teicoplanin potently blocks the cell entry of 2019-nCoV. BioRxiv. (2020).
56. Zhang, J.J., Dong, X., Cao, Y.Y., Yuan, Y.D., Yang, Y.B., & Yan, Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020).
57. Zheng, J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 16, Page (2020).
Received: 26 Aug. 2020
Accepted: 28 Sept. 2020
Ameneh Norouzi1*
1Master of Cellular and Molecular Biology, Division of Biochemistry, Iran.
*Corresponding author: Ameneh Norouzi (BSc, MSc), Master of Cellular and Molecular Biology, Division of Biochemistry, Iran. Email address: Amen.norouzi@yahoo.com.



















