Vol 5 No 1 2020 – 7
INVESTIGATION / RESEARCH
Evaluation of Gene Variants in TGFB1, SERPINF1 and MEPE in a Spanish Family Affected by Otosclerosis and Tinnitus
Francisco J. Álvarez1, Santiago Álvarez4, Jesús Alonso3, Pedro García2
Available from: http://dx.doi.org/10.21931/RB/20120.05.01.7
ABSTRACT
Otosclerosis (OTSC) is a common type of deafness affecting up to 0.4 % of Caucasians. Its familial form is inherited in an autosomal dominant fashion, although to this date, no definitive cause for OTSC has been found. In the development of OTSC, three recent genetic association studies have suggested the participation of particular point mutations and small indels in the TGFB1, SERPINF1, and MEPE genes. Consequently, replicative studies are needed to confirm the role of the proposed mutations in OTSC patients. The goal of this study was to test the presence of the candidate variants described in the genes TGFB1, SERPINF1, and MEPE in a new case of familial OTSC with seven affected individuals. DNA was extracted from saliva samples of a Spanish family with several members affected by OTSC. PCR amplified target regions of some candidate genes, and the products were purified, Sanger-sequenced, and analyzed in silico. The family subject of the study did not carry the candidate variants for OTSC described in the genes TGFB1, SERPINF1, and MEPE, although it cannot be ruled out the involvement of other mutations in genes related to their same signaling pathways. This result highlights the importance of performing replicative studies for complex diseases, such as OTCS, in families of diverse origins. Additionally, a significant association of subjective tinnitus with OTSC has been found in this family, although the link between the two pathologies should be studied further.
Keywords: Otosclerosis (OTSC); Hearing Loss (HL); Tinnitus; TGFB1; SERPINF1; MEPE
INTRODUCTION
Otosclerosis (OTSC) is a complex conductive hearing loss that affects 0.3-0.4% of people of Caucasian origin and is rare among Blacks, Asians, and Native Americans 1,2,3. The disease begins around the age of 30 of average, although it can also affect individuals from the first to the fifth decade of age 4. Most patients (70-80%) suffer bilateral OTSC, and women are more affected than men in a ratio of up to 2:1 5. The disease is caused by an abnormal bone remodeling of the otic capsule, which in normal conditions does not undergo remodeling after development. This might, over time, create a deficit in the hearing threshold of air conduction, but not in bone conduction of the sound transmission 6. Although an audiometric analysis currently determines this, the most reliable diagnosis for OTSC is the stapes surgery, which is the first-line treatment for OTSC patients. This involves the replacement of the defective stapes bone with a micro-prosthetic device 7,8.
The etiology of OTSC is not well understood; however, it is deemed to be both genetic and environmental. The environmental risk factors include measles virus infection, use of oral contraceptives, and low sodium fluoride content in drinking water, all of them still controversial3. Regarding the genetic factors, studies on large families with many affected individuals have revealed that OTSC has an autosomal dominant mode of inheritance with a reduced penetrance of about 40% (reviewed in 9,3). Linkage analysis studies on large families have identified 8 monogenic loci associated with the disease (OTSC1-5, OTSC7-8 and OTSC10) 10,11,12,13,14,15,16,17. To date, no otosclerosis-causing genes have yet been identified in these loci 3,18.
Recent gene expression analysis and genetic association studies have suggested candidate genes for OTSC mapped outside the 8 loci identified by linkage analysis. As an example, several studies have revealed a role for the TGF beta pathway in OTSC. Particularly, the Transforming Growth Factor beta1 protein, coded by the gene TGFB1, has been suggested to play a role in the development of the disease 9,19,20,21,22,23,24. Particularly, a recent genetic association study of several gene variants of TGFB1, found the c.-509C («wild type») allele associated with normal hearing, while the c.-509T allele was associated with OTSC 24. Another candidate gene that has been recently suggested as causative of OTSC is the Serpin Family F Member 1 (SERPINF1), coding for a potent inhibitor of angiogenesis and a neurotrophic factor. Ziff et al. (2016) found a primary SERPINF1 transcript expressed in the stapes bone, (isoform 2 or SERPINF1-012) and six additional rare variants in isoform 1 (SERPINF1-001), three of them predicted to be deleterious for its function and also to affect the expression of isoform 2 transcripts 25.
The most recent candidate gene to cause OTSC is MEPE, which codes for an extracellular matrix protein belonging to the SIBLING family of secreted phosphoproteins. A functional MEPE protein participates in bone homeostasis, preventing the maturation of osteoclasts and inhibiting bone mineralization 26. Schrauwen et al. (2019) found an association of MEPE and OTSC in a study of a Turkish family affected by hereditary congenital facial paresis (HCFP) and mixed hearing loss (HL). Exome sequencing revealed variants predicted to produce truncated MEPE proteins that would increase bone remodeling in the otic capsule, compared to normal developmental conditions 27.
Tinnitus is an additional hearing condition affecting about 50% of OTSC patients 9,28 and up to 15% of the global population 29,30. The hearing of phantom sounds characterizes it, usually localized to one or both ears, but can also be felt centrally within the head. Tinnitus can be a very distressing, even disabling condition for its continuous noise perceived as a buzzing, hissing, beeping or ringing, and it becomes chronic when lasting more than a year 31,32. Tinnitus can be classified as objective tinnitus when the body generates the sound, and the examiner can hear it or as subjective tinnitus, more commonly found, if there is not a specific sound source within the body. Its etiology can be environmental, due to exposure to ototoxic drugs, head trauma, noise exposure or infections. Recently, twin studies have determined a possible genetic component with a heritability of 0.68 33,34.
In the present study, a new pedigree representing four generations of a Spanish family with a history of OTSC has been constructed. DNA samples from the core family of the proband were used to test the involvement of recently proposed gene variants of TGFB1, SERPINF1, and MEPE in the development of OTSC in that family. Besides, information about tinnitus causes -a condition of significant distress for many subjects of the family- was gathered, and interesting associations between the two conditions were found.
MATERIALS AND METHODS
Subjects: participants belonging to a Spanish family with several members affected by OTSC were recruited with informed consent. Every effort was made to keep the family name confidential. All procedures were approved by the Ethics Committee of the University of León (ETICA-ULE-023-2019) and in agreement with the Helsinki Declaration (JAMA 2000) 35. Confirmation of diagnosis was initially achieved by documented evidence of stapes surgery. Additional OTSC cases were confirmed by audiometry.
Construction of the family pedigree. Information about HL and tinnitus was gathered through interviews in the period 2017-2019. An online survey was also conducted to gather information concerning the incidence of tinnitus in the family. HL among deceased individuals was confirmed when several relatives commented positively on their condition. To confirm OTSC, clinical records of both living and deceased individuals who underwent stapes corrective surgery were obtained from the same hospital they were treated upon formal request. Most of the individuals of Generation IV or V have not been depicted because either they were not coming to age as to participate in the study, they were adults without HL symptoms or their parents were not affected by HL. Individuals II:8 and III:3 underwent corrective surgery, but we were not able to find their medical history. The family pedigree chart was created with PowerPoint (Microsoft©).
Audiometry. Standard pure tone audiometry at frequencies of 0.25-8 MHz and tympanometry plus the examination by an ear, nose, and throat (ENT) specialist were performed to confirm OTSC in individuals of the family who reported any hearing problem and in siblings of individuals with confirmed OTSC.
DNA extraction. Participating individuals were asked to provide a saliva sample. These were obtained after a one-minute mouth rinse with saline solution and kept cold till the time of genomic DNA isolation. This was achieved by standard phenol/chloroform method as in Lum and Le Marchand (1998) 36, with few modifications. The quality of the DNA was confirmed in a Nanodrop apparatus (Thermo Fisher).
DNA amplification and sequencing. Target sequences of the genes SERPINF1, TGFB1 and MEPE were amplified by PCR with primers shown in Supplementary Table 1. PCR amplified DNA sequences from both affected individuals and controls from genomic DNA and the sequence of the amplicons determined by the Sanger method and capillary electrophoresis (MegaBACE 500, Amersham Biosciences) with the same primers used for DNA amplification. DNA alignments were performed in MEGA (Molecular Evolutionary Genetics Analysis) software (https://www.megasoftware.net/) 37.
Statistical analysis. To test the independence between the OTSC and tinnitus conditions, a 2 x 2 contingency table was generated sorting selected individuals of the pedigree represented in Figure 1 into each of the following four groups: having or not OTSC, and suffering or not tinnitus. Individuals with undetermined hearing loss conditions were not included in any of the groups. Finally, a two-tailed Fisher exact probability test was applied.
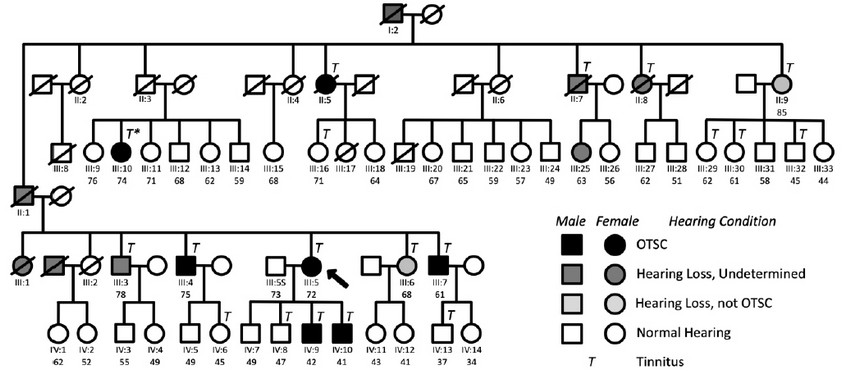
Figure 1. Genealogy of the family subject of the study showing the segregation of OTSC and chronic subjective tinnitus. See legend for conditions. An asterisk in individual III:10 indicates that her tinnitus condition disappeared upon stapes surgery. Arrow, proband.
RESULTS
Pedigree analysis
The pedigree of this study (Figure 1) contains 73 individuals of a Spanish family, including seven confirmed cases of OTSC. Not all Generation IV subjects are depicted. Generation V has been omitted because either the immediate ascendants did not suffer HL or because they were too young to be included in the study. Table 1 summarizes relevant information from individuals of the family with confirmed OTSC.

Table 1. Age, affected ear and age of earliest symptoms, diagnosis and surgery of OTSC in affected individuals. D: deceased; NA: not available; L: left ear; R: right ear. Sym/Diag: age of earliest symptoms or OTSC diagnosis.
The proband of the study (III:5, arrow in Fig. 1) was a 72-year old Spanish woman who was diagnosed with bilateral OTSC at the age of 20, three years before childbirth. She had (failed) stapes surgery in both ears. She was diagnosed also with osteoarthritis, and never took oral contraceptives. Her two youngest sons (IV:9 and IV:10) were diagnosed with unilateral OTSC and underwent a stapedectomy. Subject III:4 suffers from Paget’s disease. The medical history of subject II:5 confirmed stapedectomy. That meant individual I:2 or his spouse must have been carriers of the mutation(s) that caused otosclerosis in some of their descendants.
It was found a high prevalence of chronic subjective tinnitus in the family. Hence, participants were asked to answer a detailed questionnaire on their tinnitus experience, as in Langguth et al. (2006) 38. The results of the survey are provided in Supplementary Table 2. As shown in Figure 1, all OTSC-affected members of the family suffered from chronic subjective tinnitus. Also, reports from the interviews indicate that the tinnitus experienced by OTSC-affected subjects was severe. Interestingly, tinnitus was also present in non-affected individuals II:9, III:6, III:16, III:29, III:30, III:32, IV:6, IV:8, and IV:13 of the family. The tinnitus in individual III:10 disappeared after corrective surgery.
Genotype analysis of TGFB1, SERPINF1, and MEPE
In the present study, the contribution of previously described mutations in the genes TGFB1, SERPINF1, and MEPE was analyzed. Genomic DNA samples of the core family of the proband were used because they comprise both confirmed OTSC cases and controls. We worked on the assumption that variations found on the core family of the proband would be representative of those affecting close relatives within the family.
TGFB1. The TGFB1 allele c.-509C has been associated with normal hearing and c.-509T with OTSC 24. In the present study, we decided to determine whether SNP c.-509C > T was present in individuals affected by OTSC using genomic DNA from the core family of the proband. As shown in Figure 2, there were no differences between the sequences of affected and unaffected individuals. All members of the family carry the c.-509C allele. To note, it is the Reference Sequence Gene that has a T in that position.

Figure 2. Genotypes of the core family of the proband for the geneTGFB1. Subjects III:5S and IV:8, unaffected; III:5 and IV:9, affected. Arrows indicate the sites of the variants found in earlier studies. Image is a screen capture of alignments performed in MEGA 37.
SERPINF1. In a recent study, Ziff et al. (2016) 25 described three variants in the 5′ UTR of SERPINF1-012 (c.-202_-200 del TCG, c.-161G > C and c.-1G > C), reportedly affecting the expression of the transcript in the otic capsule. In our work we PCR-amplified and Sanger-sequenced the region comprising those variants. Figure 3 shows that none of the variants are present in the OTSC-affected individuals of the core family of the proband.

Figure 3. Genotypes of the core family of the proband for the 5’UTR region of the gene SERPINF1, isoform 2. Subjects III:5S and IV:8, unaffected; III:5 and IV:9, affected. Arrows indicate the sites of the variants found in earlier studies. Individuals III:5S, IV:8 and IV:9 are heterozygous (C/T) for the position c.-202. Images are screen captures of alignments performed in MEGA 37.
MEPE. The most recent candidate in the literature to cause OTSC is MEPE 27. Variants of this gene were found first in a Turkish family affected by HCFP and HL and later in a larger pool of OTSC subjects. In the present study none of the family members have HCFP. In our work, we amplified exons 2-4 of MEPE and adjacent regions in seven different PCR reactions using the same primers as in Schrauwen et al. (2019) 27 and DNA from individuals of the core family of the proband to look for the published gene variants -c.199_202 del GAAA (M2); c.49_54+delinsCA (M3); c.184G > T (M4); c. 496 dup (M5); c.617del G (M6) and c.679dup (M7)- and also to look for new variants. As shown in Figure 4, none of the published gene variants nor any other polymorphism were found to affect exclusively OTSC subjects.

Figure 4. Genotypes of the core family of the proband for MEPE. Arrows indicate the sites of variants named M2-M7 as in Schrauwen (2018). Subjects III:5S and IV:8, unaffected; III:5 and IV:9, affected. Images are screen captures of alignments performed in MEGA 37.
Association of OTSC with tinnitus. To find out whether OTSC and tinnitus were associated, individuals of the pedigree were sorted into four categories: not suffering tinnitus nor OTSC 48 not suffering tinnitus but suffering OTSC (0); suffering tinnitus but not OTSC 9 and suffering tinnitus and OTSC 7. Individuals with undetermined HL were not included in any of the groups. A two-tailed Fisher exact probability test indicated a very low probability that the two conditions were independent (p < 0.0001).
DISCUSSION
In the present study, a novel familial case of OTSC has been presented and used it as a tool to assess the contribution of gene variants of TGFB1, SERPINF1, and MEPE. These genes were selected because they had been recently associated with OTSC in previous reports 24,25,27. Also, they are also involved in different routes to increase bone remodeling and bone deposition in the otic capsule compared to normal conditions. Despite the available knowledge and having used the same primers and conditions for the Sanger sequencing of the genes, the genetic analysis did not find a role for those variants. This does not rule out the possibility that mutations affecting other members of the pathways to which the genes TGFB1, SERPINF1, and MEPE belong could be involved. For instance, the TGFbeta pathway has been suggested for more than a decade as involved in bone regulation at the otic capsule and it is possible that variants in other members of the TGFbeta family of cytokines like BMP2 and BMP4 could be implicated and the subject of future replicative studies 23. In the case of SERPINF1, the present work is another unsuccessful attempt at replicating the findings of Ziff et al. (2016) 25, after another research group did not find a role for the same gene variants 39.
The origin of the families participating in genetic studies may be a factor to take into account in familial OTSC cases. It cannot be excluded from the possibility that different disease-causing variants in the same genes or signaling pathways may act in families of diverse ethnic origin. One precedent is the study of Rodríguez et al. (2004) 40, which did not find a role in a Spanish family with a familial case of OTSC for COL1A1 and COL1A2, previously associated to OTSC in a family from Iowa, USA 3,41. Two other cases involving families from very different geographical locations as tools for replication studies of HL genes -although not for OTSC- analyzed the presence of the A1555G mtDNA mutation combined with suspected exposure to aminoglycosides 42,43. That mutation had been previously determined in Arab-Israeli populations 44 and was not found in Spanish families.
Reports of chronic subjective tinnitus were found in many members of the family subject of this study. Tinnitus usually affects about half of OTSC patients 9,28, and the symptoms disappear in many patients after stapes surgery -reviewed in Haider et al. (2018) 45. In the present study, 100% of OTSC-affected subjects suffered from severe forms of subjective tinnitus and only in one case (III:10) it disappeared after stapes surgery. Recently, it has been suggested a genetic basis for tinnitus 33,34. A statistical test indicated a powerful association between OTSC and tinnitus in the family subject of the study.
In conclusion, the present work has investigated the role of variants putative of causing OTSC in the genes TGFB1, SERPINF1 and MEPE, previously reported by others. Although it was not found a purpose for those mutations, further work must be done to analyze the role of variants affecting other genes belonging to the same signaling pathways. Finally, it was found a strong association of clinical OTSC with severe cases of subjective tinnitus and also many examples of tinnitus in the absence of OTSC symptoms, all within the same family. This represents an excellent opportunity to further investigate the segregation of OTSC and tinnitus with the use of genomic tools such as whole-exome sequencing of affected individuals. Finding the causative genes for OTSC and tinnitus will surely streamline the design of novel, innovative research, and therapeutic approaches for the benefit of a significant number of affected individuals.
Supplementary Material
The following information will be supplemental to this article: sequences of primers utilized in this study and Table 3 of self-reports on tinnitus.
Conflict of Interest
The authors declare no conflict of interest.
Contributions
Study design: F.J.A., P.G. Fieldwork: F.J.A., S.A. Data Collection, and Analysis: F.J.A., S.A., J.A. Sample processing: F.J.A. Data analysis: F.J.A., P.G., J.A. Scientific writing: F.J.A., P.G.
Ethical approval
“All procedures performed in studies involving human participants were following the ethical standards of the institutional Research Ethics Committee of the University of León (ETICA-ULE-023-2019) and with the Helsinki declaration of 1964 and its later amendments or comparable ethical standards.”
Funding
This work was financially supported by the Area of Genetics of the Department of Molecular Biology of the University of León in Spain.
Acknowledgments
We are very grateful to all the members of the family subject of this study for their kind and enthusiastic participation. We extend our thanks to the ENT personnel and clinical documentation services of the Complejo Asistencial Universitario de León (CAULE) for their diligent work.
REFERENCES
1. Altmann F, Glasgold A, Macduff JP. The incidence of otosclerosis as related to race and sex. Ann Otol Rhinol Laryngol 1967;76:377–92.
2. Tato JM, Tato JM Jr. Otosclerosis and races. Ann Otol Rhinol Laryngol 1967;76:1018-25.
3. Babcock TA, Liu XZ. Otosclerosis: From Genetics to Molecular Biology. Otolaryngol Clin North Am 2018;51:305-18.
4. Morrison AW. Genetic factors in otosclerosis. Ann R Coll Surg Engl 1967;41:202-37.
5. Cawthorne T. Otosclerosis. J Laryngol Otol 1955;69:437-56.
6. Batson L, Rizzolo D. Otosclerosis: An update on diagnosis and treatment. JAAPA 2017;30:17-22.
7. Abdurehim Y, Lehmann A, Zeitouni AG. Stapedotomy vs cochlear implantation for advanced otosclerosis: Systematic review and meta-analysis. Otolaryngol Head Neck Surg 2016;155:764-70.
8. Eshraghi AA, Ila K, Ocak E, et al. Advanced otosclerosis: Stapes surgery or cochlear implantation? Otolaryngol Clin North Am 2018;51:429-40.
9. Thys M, Van Camp G. Genetics of otosclerosis. Otol Neurotol 2009;30:1021-32.
10. Tomek MS, Brown MR, Mani SR, et al. Localization of a gene for otosclerosis to chromosome 15q25-q26. Hum Mol Genet 1998;7:285–90.
11. Van Den Bogaert K, Govaerts PJ, Schatteman I, et al. A second gene for otosclerosis, OTSC2, maps to chromosome 7q34-36. Am J Hum Genet 2001;68:495–500.
12. Chen W, Campbell CA, Green GE, et al. Linkage of otosclerosis to a third locus (OTSC3) on human chromosome 6p21.3-22.3. J Med Genet 2002;39:473–7.
13. Browenstein Z, Goldfarb A, Levi H, et al. Chromosomal mapping and phenotype characterization of hereditary otosclerosis linked to the OTSC4 locus. Arch Otolaryngol Head Neck Surg 2006;132:416–24.
14. Van Den Bogaert K, De Leenheer EM, Chen W, et al. A fifth locus for otosclerosis, OTSC5, maps to chromosome 3q22-24. J Med Genet 2004;41:450–3.
15. Thys M, Van Den Bogaert K, Iliadou V, et al. A seventh locus for otosclerosis, OTSC7, maps to chromosome 6q13-16.1. Eur J Hum Genet 2007;15:362–8.
16. Bel Hadji Ali I, Thys M, Beltaief N, et al. A new locus for otosclerosis, OTSC8, maps to the pericentometric region of chromosome 9. Hum Genet 2008;123:267–72.
17. Schrauwen I, Weegerink NJ, Fransen E, et al. A new locus for otosclerosis, OTSC10, maps to chromosome 1q41-44. Clin Genet 2011;79:495–7.
18. Ealy M, Smith RJH. The genetics of otosclerosis. Hear Res 2010;266:70–4.
19. Sommen M, Van Camp G, Liktor B, et al. Genetic association analysis in a clinically and histologically confirmed otosclerosis population confirms association with the TGFB1 gene but suggests an association of the RELN gene with a clinically indistinguishable otosclerosis-like phenotype. Otol Neurotol 2014;35(6):1058-64.
20. Mowat AJ, Crompton M, Ziff JL, et al. Evidence of distinct RELN and TGFB1 genetic associations in familial and non-familial otosclerosis in a British population. Hum Genet 2018;137:357-63.
21. Thys M, Schrauwen I, Vanderstraeten K, et al. The coding polymorphism T263I in TGF-beta1 is associated with otosclerosis in two independent populations. Hum Mol Genet 2007;16:2021-30.
22. Ealy M, Chen W, Ryu GY, et al. Gene expression analysis of human otosclerotic stapedial footplates. Hear Res 2008;240:80–6.
23. Schrauwen I, Thys M, Vanderstraeten K, et al. Association of bone morphogenetic proteins with otosclerosis. J Bone Miner Res 2008;23:507-16.
24. Priyadarshi S, Ray CS, Panda KC, et al. Genetic association and gene expression profiles of TGFB1 and the contribution of TGFB1 to otosclerosis susceptibility. J Bone Miner Res 2013;28:2490-7.
25. Ziff JL, Crompton M, Powell HRF, et al. Mutations and altered expression of SERPINF1 in patients with familial otosclerosis. Hum Mol Genet 2016;25:2393-403.
26. Martin A, David V, Laurence JS, et al. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 2008;149:1757-72.
27. Schrauwen I, Valgaeren H, Tomas-Roca L, et al. Variants affecting diverse domains of MEPE are associated with two distinct bone disorders, a craniofacial bone defect and otosclerosis. Genet Med 2019;21:1199-208.
28. Gristwood RE, Venables WN. Otosclerosis and chronic tinnitus. Ann Otol Rhinol Laryngol 2003;112:398-403.
29. Langguth B, Kreuzer PM, Kleinjung T, et al. Tinnitus: Causes and clinical management. Lancet Neurol 2013;12:920–30.
30. Baguley D, McFerran D, Hall D. Tinnitus. Lancet 2013;382:1600-7.
31. Vona B, Nanda I, Shehata-Dieler W, et al. Genetics of tinnitus: Still in its infancy. Front Neurosci 2017;11:236.
32. Watts EJ, Fackrell K, Smith S, et al. Why is tinnitus a problem? A qualitative analysis of problems reported by tinnitus patients. Trends Hear 2018;22:2331216518812250.
33. Maas IL, Brüggemann P, Requena T, et al. Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet Med 2017;19:1007-12.
34. Cederroth CR, Kähler AK, Sullivan PF, et al. Genetics of tinnitus: Time to biobank phantom sounds. Front Genet 2017;8:110.
35. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000;284:3043-5.
36. Lum A, Le Marchand L. A simple mouthwash method for obtaining genomic DNA for epidemiological studies. Cancer Epidemiol Biomarkers Prev 1998;7:719-24.
37. Kumar S, Stecher G, Li M, et al. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 2018;35: 1547-49.
38. Langguth B, Goodey R, Azevedo A, et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog Brain Res 2007;166:525-36.
39. Valgaeren H, Sommen M, Beyens M, et al. Insufficient evidence for a role of SERPINF1 in otosclerosis. Mol Genet Genomics 2019;294:1001-6.
40. Rodríguez L, Rodríguez S, Hermida J, et al. Proposed association between the COL1A1 and COL1A2 genes and otosclerosis is not supported by a case-control study in Spain. Am J Med Genet A 2004;128A:19-22.
41. McKenna MJ, Kristiansen AG, Bartley ML, et al. Association of COL1A1 and otosclerosis: Evidence for a shared genetic etiology with mild osteogenesis imperfecta. Am J Otol 1998;19:604-10.
42. Estivill X, Govea N, Barceló E, et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet 1998;62:27-35.
43. Morales Angulo C, Gallo-Terán J, Señaris B, et al. Prevalence of the A1555G MTDNA mutation in sporadic hearing-impaired patients without known history of aminoglycoside treatment [in Spanish]. Acta Otorrinolaringol Esp 2011;62:83-6.
44. Prezant TR, Agapian JV, Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 1993;4:289-94.
45. Haider HF, Bojić T, Ribeiro SF, et al (2018) Pathophysiology of subjective tinnitus: Triggers and maintenance. Front Neurosci 12:866.
Received: 10 january 2020
Accepted: 03 february 2020
Francisco J. Álvarez1, Santiago Álvarez4, Jesús Alonso3, Pedro García2
Corresponding autor 1. Francisco Javier Álvarez, Ph.D.
Departamento de Biología
Escuela de Ciencias Biológicas e Ingeniería
Universidad Yachay Tech
Urcuquí, Imbabura
100115 Ecuador
Tel.: +593 062 999 500 Ext. 2626
E-mail: falvarez@yachaytech.edu.ec
ORCID ID: 0000-0003-1632-1792
2. Pedro García, Ph.D.
Área de Genética
Departamento de Biología Molecular
Universidad de León
24071 León, Spain
Tel.: +34 987 291 548
E-mail: pgarg@unileon.es
ORCID ID: 0000-0002-3800-7326
3. Jesús Alonso, M.D.
Servicio de ORL
Complejo Asistencial Universitario de León
C/Altos de Nava s/n
24080 León, Spain
Tel.: +34 987 237 400 ext. 43262
E-mail: jealonsoalonso@hotmail.com
4. Santiago Álvarez
External collaborator
Valverde de la Virgen
4198 León, Spain
Tel.: +34 688998869
E-mail: unicooners@gmail.com



















