Vol 8 No 2 2023 – 22

Biosynthetic of Green Zinc Oxide Nanoparticles with Effect on Cancer Cell Line Hela
1 Biology Department, College of Education for Pure Science, University of Diyala / Iraq Email:mohanad@uodiyala.edu.iq, mohanadwm888@gmail.com
2 Biology Department, College of Education for Pure Science, University of Diyala / Iraq Email: agele.1993@gmail.com
* Correspondence: Email:mohanad@uodiyala.edu.iq, Tel. +9647700102092, Baqubah, Diyala, Iraq MJJ2+R9G.
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.22
ABSTRACT
The cytotoxic impact of biosynthetic zinc oxide nanoparticles (ZnONPs) was investigated using Vitex agnus-castus, which has been shown to have Effective compounds that suppress cancer cell proliferation. Zinc oxide nanoparticles were biosynthesized in the laboratories of the Biology department /College of Education for the Pure Sciences /University of Diyala. The phenotypic and structural characteristics of biosynthetic nanoparticles were identified using scanning electron microscopy (SEM). The majority of ZnONPs are dense and spherical in shape, with diameters ranging from (20-61) nm, and were discovered on the cervical cancer cell line Hela and compared to the normal line Human Foreskin Fibroblast cells (HFF) using the MTT stain test (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium ). Cytotoxicity experiments were conducted at the Iraqi Center for Cancer Research / Al-Mustansiriya University. This study showed inhibitory activity on Hela cervical cancer cells, where the highest inhibition rate reached 93.6% at a concentration of 200 µg/ml. This raises the prospect of finding a viable therapy for cervical cancer (Hela) or any other malignancy using nanoparticle manufacturing technologies.
Keywords: Biosynthetic, Green nanoparticles, Cancer cell, Hela, GC-MS.
INTRODUCTION
Cervical cancer is the fourth most frequent disease and the top cause of death among women worldwide, despite being highly preventable. Cervical cancer is frequently the primary cause of cancer-related sickness and mortality in low-income nations1, 2.
Green nanoparticle synthesis using biological systems, mainly plant extracts, is an emerging subject in nanotechnology. Biosynthetic nanoparticles have piqued attention owing to intrinsic benefits such as speed, environmental friendliness, and cost-effectiveness3. Nanobiotechnology represents the integration of biotechnology and nanotechnology to develop green, synthetic and environmentally friendly technology for nanomaterials formulations 4,5. Advances in using metal oxide nanoparticles in environmental applications have stimulated the need to synthesize them using green chemistry techniques across ecologically sensitive biological systems 6,7.
Synthesis of ZnONPs nanoparticles from biological pathways is preferred due to the natural bio-reducing property that reduces the use of toxic chemicals and their exposure to the environment when compared with physical and chemical methods8. Among the various inorganic nanoparticles, ZnONPs are attracting more and more attention due to their large bandwidth, high excitation binding energy, simplicity, easy manufacturing, biologically safe, non-toxic, biocompatible, eco-friendly and eco-friendly. Zinc oxide nanoparticles are readily soluble in biological fluids and assemble quickly under different physiological conditions 9.
The effectiveness of biosynthesized ZnONPs as an anticancer agent is dose-dependent, which means that increasing the concentration of ZnONPs increases its efficacy against cancer cells it does not affect normal cells. Using nanoparticles for targeted drug delivery has been an exciting research opportunity for effective cancer treatment. Targeted drug delivery to cancer cells will reduce drug dose for treatment and its other side effects10. Nanotechnology in this field aims to develop the therapeutic effect of drug molecules, where drug delivery methods are of great importance in medicine because it achieves more significant success in influencing the biological activity of the cell and the genetic material as well as gene expression 11,12,13.
MATERIALS AND METHODS
Extract preparation
Vitex agnus-castus was prepared from Krayat nurseries in Baghdad governorate and planted in the house garden. The species was confirmed by classifying it by the botanical herbarium at the College of Science / University of Baghdad, based on the book Flora of Iraq. The leaves were collected and washed with distilled water to remove particulate matter, then dried at room temperature for a week. The dried leaves were ground into a fine powder, and the extract was prepared by boiling 25 g of the powder in 250 ml of distilled water for 15 minutes. Then the extract was filtered using NO: 0.1 filter paper. The filtered extract was stored in a refrigerator at 4°C for later use in the biosynthesis of ZnONPs. Also, an alcoholic extract of the plant was prepared for the detection of the active compounds by using a gas chromatography-integrated mass spectrometer (GC-MS) device for the alcoholic extract of leaves. The leaves were extracted using the saxolith system and using hexane and methanol as solvents, respectively, according to method 14 with some modifications. 100 g of leaf powder was placed in a filter paper made in the form of a funnel and closed to avoid the exit of the vegetable powder. Then it is placed in a saxolithic apparatus, and 500 ml of solvent is placed on it for 24 hours. Then the solution was concentrated by a rotary evaporator, and the solvent was separated, as a dark brown oil was produced.
Biosynthesis of (ZnONPs)
According to a method described by Pillai et al. (2020), modifications were made to 100 ml of the previously prepared plant extract heated to 60-70°C on a magnetic stirrer. When the temperature reaches 60 degrees Celsius, 10 grams of zinc nitrate Zn(NO3)2 is added. It is boiled until it turns into a creamy white paste. After that, the dough is washed with distilled water and placed in a hot oven at 400 ° C for two hours. Then a white powder will be formed from ZnONPs loaded with active compounds from the plant extract 15, as shown in Figure (1).
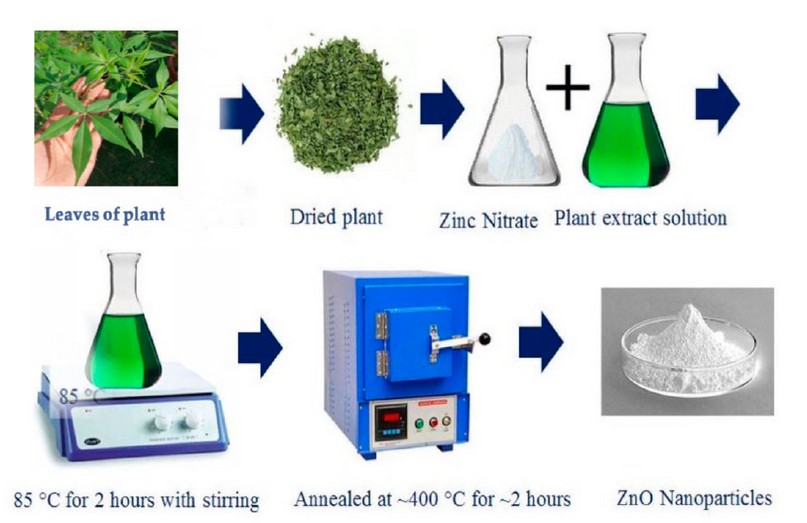
Figure 1. Biosynthesis of zinc oxide nanoparticles 16.
Scanning Electron Microscope (SEM)
The biosynthetic ZnONPs assays were carried out to show the phenotypic and structural properties of the biosynthetic particles using scanning electron microscopy (SEM) at Kashan University – Iran. The device works on imaging samples with a magnification of 25-250000 times and scans samples with very high accuracy. The focused beam interacts with the samples by means of electrons that collide with the sample atoms and transmit signals that evaluate the topography. The detectors turn the electron intensity into a digital voltage that is stored. Three-dimensional images are composed of a size that can measure 1 to 5 nanometers 17.
Cytotoxicity Assays
The cell lines were placed in a tissue culture vessel of 25 cm3 in size. The container includes Roswell Park Memorial Institute (RPMI-1640) culture medium and 10% calf fetal serum. The container was placed on the cell suspension and culture medium in a 5% CO2 incubator at 37 °C for 24 hours. After that, the container with the cell suspension is taken out of the incubation, and it is confirmed that there is no contamination in the cell culture through an inverted microscope. Secondary cultures are formed and left in the incubator for 24 hours, after which they are examined again with an inverted microscope to ensure their growth and the absence of contamination in the medium. Then the cells were transferred to the growth cabin, the used culture medium was emptied, and the cells were washed using Phosphate Buffer Saline. The washing process was repeated twice for 10 minutes each time. A sufficient amount of Trypsin/Versine enzyme was added to cover the cells and placed in the incubator for 30-60 seconds at a temperature of 37°C. Then it is noticed that it has transformed from a group of cells compact in one layer to single cells that are not compact after disengaging them with the tissue culture vessel. The action of the Trypsin/Versine enzyme is stopped by adding a fresh culture medium containing 10% serum. The test was performed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye 18. using 96-hole Cell culture plates, each line was cultured at 10,000 cells/pit using an automatic Micotiter plat for tissue culture.
After 24 hours, the monolayer cells were confirmed. Biosynthetic ZnONPs nanoparticles were added at various concentrations for 72 hours. After exposure, the cells were washed three times with phosphate buffer saline after the culture medium was taken out. The culture plate was then incubated at 37 °C after 30 l of MTT dye was added at a concentration of 2 mg/ml. For 3 hours, then 25 μl of DMSO (Dimethyl Sulphoxide) solution was added to each pit for 10 minutes. The culture plate was incubated again at 37°C, after which the absorbance was measured on a microplate reader (ELISA) at 492 nm. Then the percentage of cytotoxicity was calculated after that, the rate of cytotoxicity was calculated using the following equation.
Cytotoxicity killing % = CO.O mean – txO.Omean / CO.O mean x 100
where :
CO.O mean: Optical density of cells that are not treated with nanomaterial
txO.Omean: Optical density of cells which are treated with nanomaterials
Statistical Analysis
The data attained by using the unpaired t-test were analyzed using the statistical software (Graphpad Prism 6). The values were estimated as (arithmetic mean ± standard error) for the three replicates with a significant difference at the significance level P < 0.05 according to the statistical package used in 19.
RESULTS
GC-Mass assay results for essential oils
The individual components of the leaf extract were identified by computer matching with the commercial Wiley GC/MS mass spectral libraries, the Mass Finder 3 library, and the Baser library for the essential oil components which includes more than 3,200 authentic compounds with mass spectra and retention data of pure oil. The results appeared in the presence of active compounds in the extract as shown in Table (1).
Table 1. Shows the active compounds of Vitex agnus-castus leaf extract in the GC-Mass system.
(SEM) Scanning Electron Microscope
The surface morphology of the biosynthesized ZnONPs was characterized using (SEM) as in Fig. (2). The image has a magnification power (150 KX). It can be seen that most of the ZnONPs are dense and spherical in shape, their sizes range between (20-61) nm.

Figure 2. SEM image showing biosynthesized ZnONPs
Cytotoxicity Experiments
The cytotoxicity of cervical cancer Hela was investigated by comparison with the normal cell line HFF using various concentrations of biosynthetic zinc oxide nanoparticles. The cytotoxicity was investigated by reading the intensity of cell uptake using an ELISA device at wavelength 492 nm and calculating the percentage change in cell growth according to the following equation:

Percentage change in growth = x 100
The toxicological effect of various concentrations of biosynthetic ZnONPs on the growth of cervical cancer Hela cells was investigated and compared with the normal HFF line by calculating the optical density (0.D) values of cell growth after an exposure period of 72 hours and at a temperature of 37 °C in three replicates with eight various concentrations: (6.25), (12.5), (25), (50), (100), (200), (400), and (800) µg/ml. Cytotoxicity test was performed using MTT stain. Depending on the results of the average percentage inhibition as well as the percentage of cell vitality as shown in Figure (3), the inhibition in the vitality of cervical Hela cells increases as the percentage of inhibition increases with the increase in the concentration of biosynthetic ZnONPs. The results are shown in Figure (4), indicating shallow inhibition values for the viability of HFF cells when treated with biosynthetic ZnONPs. Table (2) shows the percentage of cell viability inhibition at each cervical cancer Hela cell concentration and its comparison with the regular HFF line.
The IC50 half-lethal dose of biosynthetic ZnONPs on cervical cancer cells was 35.74 µg/ml as shown in Figure (5). In the cells of the normal line, half of the lethal dose IC50 was 655.7 µg/ml as shown in Figure (6).
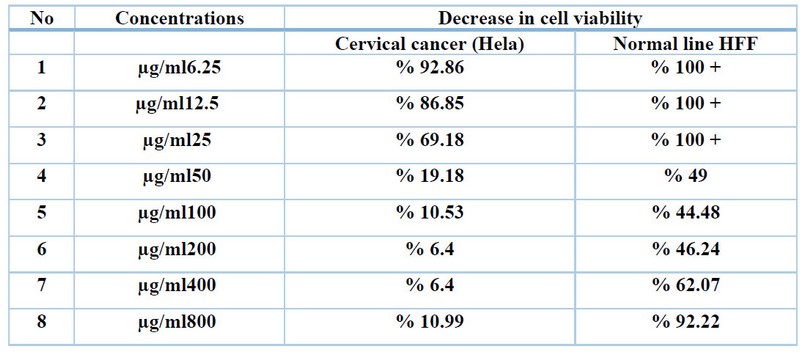
Table 2. Shows the percentage of decrease in cell viability in each cell line at different concentrations of biosynthetic ZnONPs.
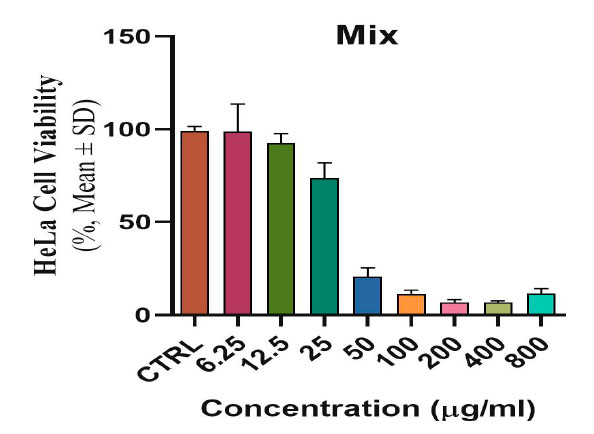
Figure 3. Shows the toxic and inhibitory effect of biosynthetic ZnONPs on cervical cancer Hela cells.
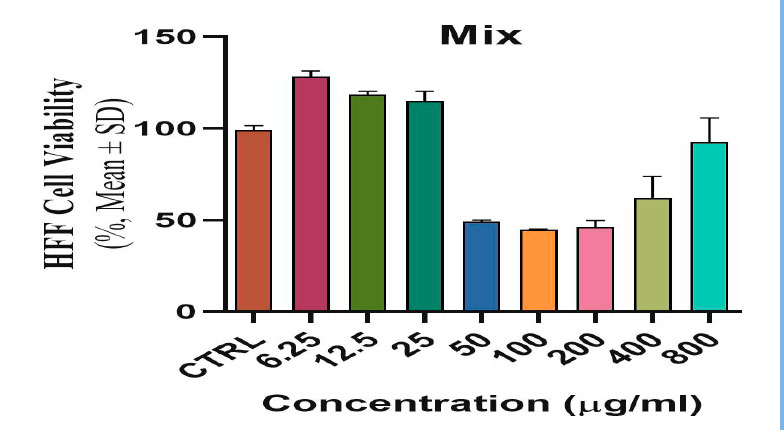
Figure 4. Shows the toxic and inhibitory effect of biosynthetic ZnONPs on HFF normal line cells.
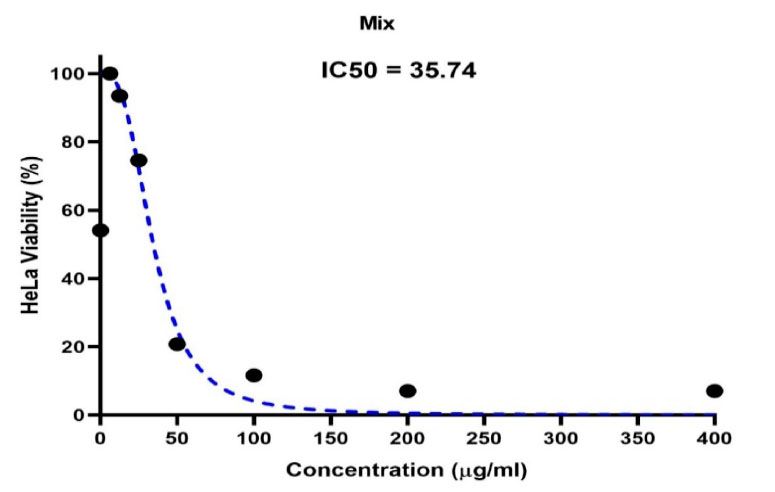
Figure 5. Shows the concentration of a half-lethal dose of biosynthetic ZnONPs on cervical cancer Hela cells.
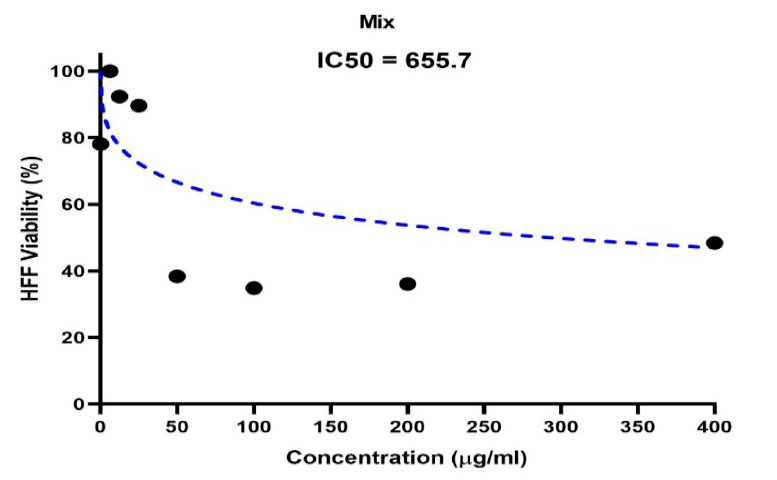
Figure 6. Shows the concentration of half the lethal dose of biosynthetic ZnONPs on normal HFF cells.
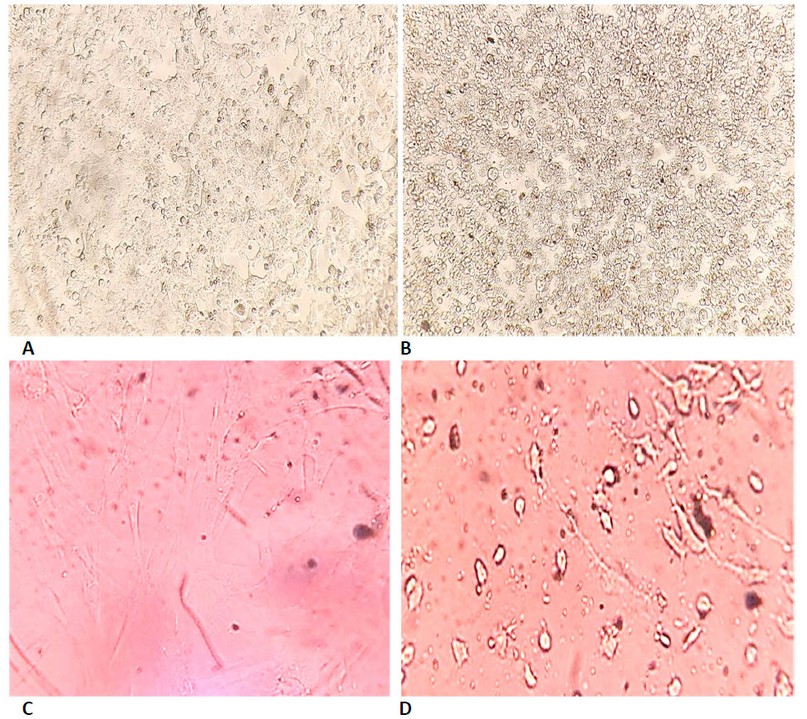
Figure 7. Shows the cytotoxicity of biosynthesized ZnONPs on cell lines using MTT dye for 72 hours at 37°C. (A) represents cervical cancer Hela cells which are not treated with biosynthetic ZnONPs. (B) represents cervical cancer Hela cells treated with biosynthetic ZnONPs at a 50 µg/ml concentration. (C) represents normal HFF cells untreated with biosynthetic ZnONPs. (D) Represents normal HFF cells treated with biosynthetic ZnONPs at a 25 µg/mL concentration.
DISCUSSION
GC-Mass assay results for essential oils were Vitex agnus – castus possesses a wide range of active chemical ingredients, including essential oils, flavonoids, iridoid glycosides, diterpenoids and phenolic compounds20.
The results of (the SEM) Scanning Electron Microscope are most of the ZnONPs are dense and spherical in shape ranging in size from (20-61) nanometers; in another study, ZnO nanoparticles were synthesized using plant extracts, the researchers observed that the SEM images at room temperature and 90 °C had 50 nm and 40 nm size and spherical morphology. 21, 22.
The results in Table (2) showed a decrease in the vitality of the cells, and this decrease is inversely related to the concentration. The higher the engagement, the lower the vitality of the cells. The first decrease in the viability of cancer cells started at a concentration of 6.25 µg/ml on cervical cancer cells. Cell viability decreased to 92.86% in cervical cancer. Cell viability continues to decline as the concentration of biosynthetic ZnONPs increases. As the cell viability reaches 10.99% in cervical cancer cells at a concentration of 800 µg/ml. In normal cells, the cell viability reached 92.22% at a concentration of 800 µg/ml. This confirms that the biosynthetic zinc oxide nanoparticles have a toxic effect on cancer cells with low impact on normal cells. This is identical to what was stated in the results of the research by Latif and Alzubaidy (2021) 23. Nanoparticles are used in nanomedicine that contribute to diagnosing and treating various diseases including cancer. The unique property of nanomedicine, that is, their high surface-to-volume ratio enables them to bind, absorb and transport small biomolecule such as DNA, RNA, drugs, proteins, and other molecules. to the target site, thus enhancing the efficacy of therapeutic agents24.
The results proved that the biosynthesized ZnONPs can cause phenotypic changes in the shape, size and number of cancer cells. Still, these changes occurred at concentrations lower than the concentrations used for the physically prepared ZnONPs, as well as a higher rate of inhibition in using the biosynthetic ZnONPs. Cells after exposure to biosynthetic ZnONPs became non-adherent single cells after they were compact cells. The differentiation of these cells is due to the death of many cells and their loss of adhesion to each other, which leads to the death of many cancer cells, as shown in Figure (7). These results prove that the biosynthetic ZnONPs are more effective and inhibiting on cancer cell lines and safer, less expensive and easier to prepare than other methods (physical or chemical). These results are consistent with the findings of studies 22-26. The cytotoxicity of biosynthetic ZnONPs may be due to the release of dissolved zinc ions within cells and the induction of reactive oxygen species (ROS). The increase in zinc ions also led to an imbalance in the activity of proteins and oxidative stress, thus killing the cell 27. The cytotoxicity of biosynthetic ZnONPs is also due to the ability of these particles to slip into the nucleus, bind to the genetic material, and cause a disruption in cell functions, leading to cell death 28.
CONCLUSIONS
The process of using plant extracts in the biosynthesis of nanoparticles had high activity in inhibiting cancer cells because of its unique properties represented by considering the active substances in the plant as a reducing agent. It can be considered as one of the most environmentally friendly methods. Biosynthesis is safer for normal, uninfected cells. This gives hope to discover a successful treatment to eliminate the most common types of cancer, including cervical cancer (Hela).
Acknowledgments
Thanks and appreciation to the Deanship of the College of Education for Pure Sciences and the Deanship of the Iraqi Institute for Cancer Research and Medical Genetics / Al-Mustansiriya University. For their help and for facilitating all the requirements for conducting the study.
REFERENCES
1. Castle, P. E., Einstein, M. H., and Sahasrabuddhe, V. V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA: a cancer journal for clinicians (2021). https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21696
2. Preciado, C., & Janeta, P. V. Bionatura Conference Series Vol 2. No 1. 2019. https://revistabionatura.org/files/CS-2019.02.01.25—Bionatura.pdf
3. Jadoun, S., Arif, R., Jangid, N. K., and Meena, R. K. . Green synthesis of nanoparticles using plant extracts: A review. Environmental Chemistry Letters(2021) , 19(1), 355-374.
https://link.springer.com/article/10.1007/s10311-020-01074-x
4. Buazar, F., Baghlani‐Nejazd, M. H., Badri, M., Kashisaz, M., Khaledi‐Nasab, A., & Kroushawi, F. Facile one‐pot phytosynthesis of magnetic nanoparticles using potato extract and their catalytic activity. Starch‐Stärke, (2016), 68(7-8), 796-804. https://onlinelibrary.wiley.com/doi/abs/10.1002/star.201500347
5. Mohanad W. Mahdi Alzubaidy. Evaluation the Breast Cancer Cell Line DNA Damage by Biosynthetic Silver-Green Nanoparticles. Texas Journal of Agriculture and Biological Sciences, (2022), 3, 14–19. https://zienjournals.com/index.php/tjabs/article/view/1254
6. Selim, Y. A., Azb, M. A., Ragab, I., & Abd El-Azim, M. H. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Scientific reports, (2020), 10 (1), 1-9.https://nature.com/articles/s41598-020-60541-1
7. Dahoumane, S. A., Mechouet, M., Alvarez, F. J., Agathos, S. N., & Jeffryes, C. (2016). Microalgae: An outstanding tool in nanotechnology. Bionatura, 1(4), 196-201. https://revistabionatura.org/files/7-Microalgae–An-outstanding-tool-in-nanotechnology.pdf
8. Aldalbahi, A., Alterary, S., Ali Abdullrahman Almoghim, R., Awad, M. A., Aldosari, N. S., Fahad Alghannam, S., … & Abdulrahman Alrashed, R. Greener synthesis of zinc oxide nanoparticles: Characterization and multifaceted applications. Molecules, (2020), 25 (18), 4198.https://mdpi.com/1420-3049/25/18/4198
9. Hamrayev, H., Shameli, K., and Yusefi, M. Preparation of zinc oxide nanoparticles and its cancer treatment effects: A review paper. Journal of Advanced Research in Micro and Nano Engineering, (2020), 2 (1), 1-11.https://akademiabaru.com/submit/index.php/armne/article/view/2962
10. Wu, H. and J. Zhang. «Chitosan-Based Zinc Oxide Nanoparticle for Enhanced Anticancer Effect in Cervical Cancer: A Physicochemical and Biological Perspective.» Saudi Pharm J 26, (2018) no: 205-10. http://dx.doi.org/10.1016/j.jsps.2017.12.010
11. Ziad T. Khodair, Mohanad W. Mahdi Alzubaidy, Asmaa M. Salih Almohaidi, Ammar Ahmed Sultan, Sana M. H. AL-Shimmary, and Sarah S. Albusultan. Synthesis of copper oxide nanoparticles (CuO-NPs) and its evaluation of antibacterial activity against P. aeruginosa biofilm gene’s. Technologies and Materials for Renewable Energy, Environment and Sustainability AIP Conf. Proc. 2190, 020006-1–020006-7; https://doi.org/10.1063/1.5138492 Published by AIP Publishing. (2019), 978-0-7354-1937-7/.
12. Mohanad W. Mahdi Alzubaidy, Asmaa M. Salih Almohaidi, Ammar Ahmed Sultan, and Sana M. H. ALShimmary. Virulence gene of Pseudomonas aeruginosa with nanoparticle. AIP Conf. Proc. 2123, 020039-1–020039-9; https://doi.org/10.1063/1.5116966 Published by AIP Publishing. (2019), 978-0-7354-1863-9/.
13. Mohanad W. Mahdi Alzubaidy. SILVER NANOPARTICLES PREPARED BY SOL-GEL METHOD EFFECT ON ESCHERICHIA COLI AS ANTIMICROBIAL. International Scientific Research Journal (WoS) Vol. 3 (2022), No. 3 https://doi.org/10.17605/OSF.IO/UCTVJ.
14. Uddin, S., Alnsour, L., Segun, P., Servi, H., Celik, S., Göktürk, R. S., … & Sarker, S. D. Flavonoids from two Turkish Centaurea species and their chemotaxonomic implications. Trends in Phytochemical Research, 1,(2017),(4), 243-248.
http://tpr.iaushahrood.ac.ir/article_535458_4bd1d338c5035fbec3291473fba50bdc.pdf
15. Pillai, A. M., Sivasankarapillai, V. S., Rahdar, A., Joseph, J., Sadeghfar, F., Rajesh, K., & Kyzas, G. Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. Journal of Molecular Structure, (2020), 1211, 128107.
https://sciencedirect.com/science/article/abs/pii/S0022286020304324
16. Barzinjy, A. A Characterization of ZnO Nanoparticles Prepared from Green Synthesis Using Euphorbia Petiolata Leaves. Eurasian Journal of Science & Engineering, (2019), 4(3), 74-83.
http://eprints.tiu.edu.iq/645/
17. Řiháček, T., Horák, M., Schachinger, T., Mika, F., Matějka, M., Krátký, S., … & Müllerová, I. Beam shaping and probe characterization in the scanning electron microscope. Ultramicroscopy, 225, (2021), 113268. https://sciencedirect.com/science/article/abs/pii/S0304399121000589
18. Wu, S., & Shah, D. K.. Determination of ADC cytotoxicity in immortalized human cell lines. In Antibody-Drug Conjugates (2020) , (pp. 329-340). Humana, New York, NY. https://link.springer.com/protocol/10.1007/978-1-4939-9929-3_23
19. Mitteer, D. R., & Greer, B. D. Using GraphPad Prism’s Heat Maps for Efficient, Fine-Grained Analyses of Single-Case Data. Behavior Analysis in Practice, (2022), 1-10.https://link.springer.com/article/10.1007/s40617-021-00664-7.
20. Ababutain, I. M., & Alghamdi, A. I. In vitro anticandidal activity and gas chromatography-mass spectrometry (GC-MS) screening of Vitex agnus-castus leaf extracts. PeerJ, 9, (2021), e10561 .https://peerj.com/articles/10561/
21. Gur, T., Meydan, I., Seckin, H., Bekmezci, M., & Sen, F. Green synthesis, characterization and bioactivity of biogenic zinc oxide nanoparticles. Environmental (2022), Research, 204, 111897. https://sciencedirect.com/science/article/abs/pii/S0013935121011920
22. Alzubadiy, M. W. M., Almohaidi, A. M. S., Sultan, A. A., & Abdulhameed, L. Q. Evaluation of E-selectin rs 5367 C/T Polymorphism in Iraqi Diabetic Foot patients. Paper presented at the Journal of Physics: Conference Series(2019). https://iopscience.iop.org/article/10.1088/1742-6596/1294/6/062021/meta
23. Latif, B. M. A., & Alzubaidy, M. W. M. Effect of Zinc Oxide Nanoparticles On Activity of Cell Line (B16) Causes Skin Cancer. NVEO-NATURAL VOLATILES & ESSENTIAL OILS Journal| NVEO, (2021), 472-478. https://nveo.org/index.php/journal/article/view/3645.
24. Chaturvedi, V. K., Singh, A., Singh, V. K., & Singh, M. P. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Current drug metabolism, (2019). 20(6), 416-429.
25. Vijayakumar, S., Chen, J., González-Sánchez, Z. I., Durán-Lara, E. F., Divya, M., Shreema, K., … & Vaseeharan, B. Anti-Colon Cancer and Antibiofilm Activities of Green Synthesized ZnO Nanoparticles Using Natural Polysaccharide Almond Gum (Prunus dulcis). Journal of Cluster Science, (2021) , 1-12. https://link.springer.com/article/10.1007/s10876-021-02205-2
26. Abdelhakim, H. K., El‐Sayed, E. R., & Rashidi, F. B.. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. Journal of Applied Microbiology, (2020), 128(6) 1634-1646. https://sfamjournals.onlinelibrary.wiley.com/doi/abs/10.1111/jam.14581.
27. Hamrayev, H., Shameli, K., & Korpayev, S.. Green Synthesis of Zinc Oxide Nanoparticles and Its Biomedical Applications: A Review. Journal of Research in Nanoscience and Nanotechnology, 1 ,(2021), (1) 62-74. https://akademiabaru.com/submit/index.php/jrnn/article/view/3595
28. Wang, J., Lee, J. S., Kim, D., & Zhu, L.. Exploration of zinc oxide nanoparticles as a multitarget and multifunctional anticancer nanomedicine. ACS applied materials & interfaces, (2017), 9(46) 39971-39984. https://pubs.acs.org/doi/abs/10.1021/acsami.7b11219.
Received: 2 January 2023/ Accepted: 19 April 2023 / Published:15 June 2023
Citation: Mohanad W. Mahdi Alzubaidy, Mohammed Nazar Hussain. Biosynthetic of Green Zinc Oxide Nanoparticles with Effect on Cancer Cell Line Hela. Revis Bionatura 2023;8 (2) 22. http://dx.doi.org/10.21931/RB/2023.08.02.22




















