Vol 8 No 4 2023 – 31
Evaluation of Calretinin and enumeration of mast cells in rectum tissue biopsies of Hirschsprung and non-Hirschsprung disease in neonate and infant
Rusul A. Abdul Hussein 1*, Sahar A. H. AL-Sharqi 2, Nada K. Mehdi3 and Ali E. Joda 4,5
1,2 Department of Biology, College of Science, Mustansiriyah University, Baghdad/Iraq
3 Histopathology Specialist, Central Child Teaching Hospital, Baghdad, Iraq.
4 Pediatrics department, College of Medicine, Mustansiriyah University, Baghdad/Iraq
5 Consultant pediatric surgeon, Central Child Teaching Hospital, Baghdad, Iraq
* Corresponding Author: Rusul A. Abdul Hussein, Email: rusul_abass@yahoo.com
Available from: http://dx.doi.org/10.21931/RB/2023.08.04.32
ABSTRACT
The Hirschsprung disease (HD) is a complex genetic congenital condition characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the colon and rectum, leading to functional intestinal obstruction. A study was conducted from July 2022 to December 2022. The Toluidine blue stain and calretinin immunohistochemistry were applied to 36 cases of neonates and infants who clinically presented with symptoms suspicious of having HD, And the hematological study of cell blood counts test and compared the result of the HD group with the non-HD group and control group. The study showed an increase in mast cell numbers in the rectal biopsy tissue of HD patients compared with non-HD patients using Toluidine blue stain. The Immunohistochemistry for calretinin result displayed 27 (75%) cases as HD, while the remaining 9 (25%) cases were confirmed as non-HD and showed hypertrophied nerve fiber in HD cases. at the same time, the complete blood count result was unrelated to HD. Some worrying maternal risk factors were highlighted during pregnancy were the age of the mother at conception, maternal illness, intake of drugs, type of Childbirth, and number of previous maternal abortions; all of them show a non-significant difference between the HD group and non-HD group, also consanguineous marriage was detected and shows a significant difference between the HD group and non-HD group.
Keywords: Hirschsprung, Calretinin, Toluidine blue, CBC count
INTRODUCTION
The Hirschsprung disease (HD) is a complex genetic congenital condition. The Latin name for HD is megacolon congenitum 1,2. According to reports, HD has a 4:1 male-to-female ratio and impacts 1 case out of every 5,000 live births globally 3,4. The gold standard for diagnosing HD is a rectal biopsy 5. If a patient’s clinical symptoms (failure to pass meconium, abdominal distension, constipation) and radiological findings raise suspicion of HD, the Rectal suction biopsy is obtained 6. Calretinin is calcium signaling that involves a binding protein 7, found in enteric neurons that project into the mucosal and submucosal layers of the gut. This protein might be used as a marker for aganglionosis in HD; it is a crucial component of how cells function and is expressed by the CALB2 gene 8. Mast cells (MCs) are immune cells that migrate from the bone marrow and perform their primary functions in various peripheral tissues 9. The proximity of MCs to nerve fibers, the possibility that they play a physiological role in nerve fiber growth and repair, and the fact that they produce, store, and release the nerve growth factor necessary for the growth and repair of nerve fibers all point suggest that MCs are to blame for the hyperplasia and hypertrophy of adrenergic and cholinergic nerve fibers, which are typical HD symptoms. However, the precise function of MCs in HD is still unknown. MCs can be seen thanks to the Toluidine Blue (TB) stain. The part mast cells play in HD has recently attracted much attention 10. Because most specialist doctors find it challenging to diagnose HD, and the different methods of diagnosis depend on clinical signs and radiological examinations.
The study aims to establish a scientific basis for diagnosing the disease based on the Evaluation of Histopathological and Immunohistochemical (IHC) changes that occur in the layers of the colon, with detecting changes that occur in blood cells and collecting, analyzing, and studying data, to specify diagnosis fundamentals.
MATERIALS AND METHODS
Subjects
The study included the histopathological, hematological, and IHC examination of 36 cases suspected of having HD. The ages of cases ranged between (1day-1year) for both sexes, 9 females and 27 males; the sample was obtained from the Central Teaching Hospital for Children and teaching laboratories at the Medical City Hospital in Baghdad from July 2022 to December 2022. All participants agreed to provide the investigator with the specimens. The College of Science, Mustansiriyah University’s ethics committee approved this work. According to the Declaration of Helsinki, informed consent was obtained from all participants (Ref. No.: BCSMU/0622/0012Z Appendix-1).
Collection and preparation of the rectum tissue specimens
The tissue specimens were obtained from the rectum of 36 neonates and infants (27 male and 9 female) who clinically presented with symptoms suspicious of having HD after being diagnosed by specialized doctors, the cases were rectal punch biopsies as shown in Figure (1), the tissue samples were maintained in the fixative solution (formalin 10%) for histopathological study.

Figure 1. Image of cases in which patients showed stages of obtaining a biopsy from the rectum.
Tissue samples from the rectal biopsy were prepared for histological study using Suvara 11. Each tissue sample was cut into small fragments about 2- 3 cm long before fixation in a buffered isotonic solution of 10% formaldehyde for 24 hours. Each biopsy was processed for the dehydration process, which was by passing them through progressive concentrations of ethanol alcohol. They were cleared by passing them through two steps of xylene. Then, tissues were infiltrated with paraffin wax and were embedded in a metal template, and after that, the paraffin blocks were sectioned by rotary microtome into sections 5 μ in thickness. After staining with TB for MC examination, the slides were examined using a light microscope.
Enumeration of Mast Cells in the Tissue
After sections were stained with TB, the grading of MCs in rectal tissue was done using the following method of Amerada et al. 12: – /+: No cells or few; +: 10 cells seen per 10 high power fields; ++: Clusters of more than 10 cells seen per 10 high power fields; +++: > 10 clusters seen in 10 per high power fields. The results were analyzed after grading.
Immunohistochemical study of Calretinin
The IHC stain for Calretinin was conducted in cases that included 36 formalin-fixed, paraffin-embedded rectal incisional biopsies from neonate and infant patients. IHC of Calretinin was graded as A total absence of staining (negative) or presence of brown staining (positive) according to the method of Leica Company from Germany.
Collection of Blood Samples
From the 36 patients and 20 controls, about 3 ml of venous blood was collected from a suitable vein and withdrawn from the cases using 3 ml disposable syringes.
The blood (3 ml) was then collected in a tube containing ethylene diamine tetra acidic acid (EDTA) as an anticoagulant with a slow mix for a hematological investigation.
Some Complete Blood Count (CBC)
In this test, 3 ml of the non-hemolyzed blood is anti-coagulated with EDTA at the collection and was examined by using an automated system Sysmex XP300 hematology analyzer, which is a computerized, highly specialized machine that counts the number of total WBCs and different types of cells such as neutrophil, lymphocyte, RBCs, HCT, and platelets in a blood sample.
Statistical analysis Statistical Package for Social Sciences (SPSS) version 21 is used to interpret the data. The information is given as a mean, standard deviation, and ranges. Frequencies and percentages are used to display categorical data. ANOVA was used to compare the tested mean Data expressed as mean± SD. Values of p>0.05 were considered statically non-significant, while p≤0.05 considered significant results.
RESULTS AND DISCUSSION
Enumeration of mast cells in the rectal biopsy tissue
Enumeration of MCs in the rectal biopsies tissue of non-HD and HD cases by using a TB special stain, table (1) represents the MCs for each HD case and non-HD case, which is divided into four categories that are (Occasional cells, Few natural cells, Moderate number cells, and clumps). In the HD case, the highest grade registered for a Moderate number of cells, while the lowest was for Occasional cells. In contrast, in the non-HD case, the highest grade was reported for Occasional cells, while the lowest was for a Moderate number of cells. That could be because MC is observed in significant amounts in the digestive tract.
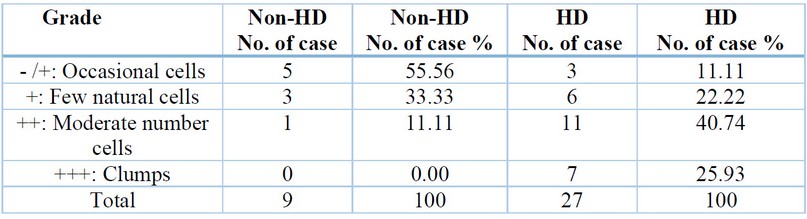
Table 1. Enumeration of mast cells in the rectal biopsies tissue of non-HD and HD cases
Results are expressed as percentage – /+: No cells or few; +: 10 cells seen per 10 high power fields; ++: Clusters of more than 10 cells seen per 10 high power fields; +++: > 10 clusters seen in 10 per high power fields In this study mast cells were seen in the submucosa and showed clump of mast cell in the muscular layer (Figure 2).
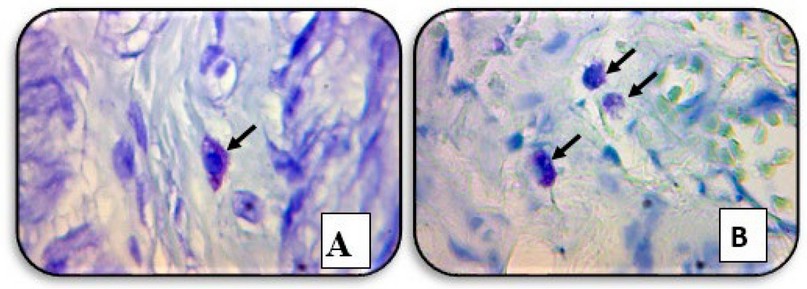
Figure 2. A cross-section of the patient’s rectal biopsy tissue showed (A) a mast cell in the submucosa. (B) a clump of mast cells in the muscular layer (black arrows) (TB staining, X40).
Recently, there has been a lot of interest in the role of MCs in HD, where TB stain highlights MCs, according to 13.. Their description of the transmural distribution of these cells in HD cases, particularly around nerve fibers and perivascular, is consistent with the findings of our study. This may be because MCs secrete a wide range of biologically active substances. MC synthesizes, stores, and releases nerve growth factor, essential for nerve fiber growth and repair 14. A similar finding was mentioned by 15. This could result from the MCs potentially having a significant impact on the regeneration and differentiation of the intestinal neural system. MCs are more prevalent in HD patients 16. However, Hermanowicz 17 carried out a study that found no statistically significant difference existed between the number of MCs in the submucosa of the HD group compared to the number of MCs in the other investigations and when comparing the mean number of MCs in the submucosa of the non-HD group in their study with a mean number of MCs in earlier 16. This study proved that the number of MCs in the submucosa of HD and non-HD groups did not differ statistically significantly. It may be caused by the MC’s reaction to allergens or pathogenic pathogens rather than by their relationship with aganglionosis 15.18. Other gastrointestinal conditions like acute appendicitis, ulcerative colitis, celiac disease, and gluten enteropathy have also been linked to an increase in MCs. In the case of suspected HD, this poses a problem for interpreting a rectal biopsy 19. However, one study done by 20 reported an increase in MCs in the mucosa of HD but no statistically significant change in the number of MCs in the submucosa, muscularis propria, and serosa; it has been discovered that numerous inflammatory illnesses at this site are connected with an increase in MCs, which are near arteries and peripheral nerves, which may be because the MCs are primarily present in the gastrointestinal tract 21, Given that many of our patients have several comorbid conditions, this may be explained by the rising number of Mcs, which may not necessarily be related to HD but may instead be caused by these conditions.22
Immunohistochemistry of Calretinin
Calretinin IHC was applied to all 36 studied cases, and 27 (75%) of the cases were identified as HD, while the remaining 9 (25%) cases were identified as non-HD Table (2). Figure (3) showed positive expression in the testis as (positive control) of IHC staining of Calretinin.

Figure 3. The immunohistochemical staining method detected Calretinin in the testis as (positive control), showing positive expression (X10).

Table 2. Expression of Calretinin in the non-HD and HD cases.
The IHC for Calretinin was applied to all 36 cases after the application of Calretinin IHC; out of 36 cases, 27 (75%) cases were confirmed as HD while the remaining 9 (25%) cases were confirmed as non-HD; hence, all suspicious HD cases had been confirmed and categorized in HD and non-HD. In our study, strong calretinin immunoreactivity was observed in all ganglionic segments (non-HD cases), figure (4), showing positive expression between the two muscularis layers and positive expression in the submucosa layer.

Figure 4. Immunohistochemical staining method detection of Calretinin in the rectal biopsies of non-HD case (A) showing positive expression between the two layers of muscularis (B) showing positive expression in the submucosa (Large figure: X10, small figure: X40)
Whereas any immunoreactivity was not observed in almost all aganglionic segments (HD cases), (Figure 5) shows a negative expression of Calretinin in the two layers of the muscularis layer while showing a complete absence of staining expression of Calretinin in the mucosa and submucosa layers.
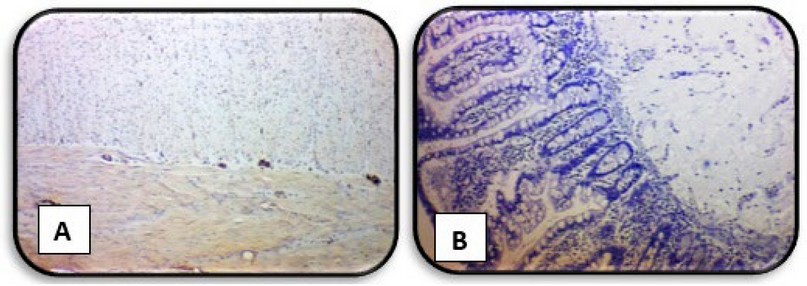
Figure 5. Immunohistochemical staining method detection of Calretinin in the rectal biopsies of HD case (A) showing negative expression of Calretinin in the two layers of muscularis layer (B) showing negative expression of Calretinin in the mucosa and submucosa layers (X4)
For HD to be pathologically diagnosed, the colonic neural plexus must be devoid of ganglion cells. Identifying tiny immature ganglion cells is made more accessible by IHC labeling of Calretinin, which causes strong ganglia staining 23. IHC expression in this study found that the calretinin IHC approach is less complicated to use, easier to interpret, and requires fewer serial sections of the microscopic rectal biopsy to detect and identify small immature ganglion cells 24. Aganglionic and ganglionic regions differed significantly from one another. Using Calretinin it was successful in detecting the presence of ganglions. The current results showed that Calretinin IHC has good diagnostic value and that Calretinin is an extremely valuable, sensitive, and specific marker for detecting aganglionosis in patients who are believed to have HD 25. This outcome is consistent with the research by 26. Barshack et al. were the first authors to report that expression of Calretinin was not observed in aganglionic areas in HD, but it was kept in ganglionic areas. They also concluded that aganglionic segments showed negative calretinin expression while positive in all rectal biopsies with ganglionic cells. Various research has reported that Calretinin is a good marker in displaying ganglia in HD, as Musa ZA et al. in Iraq revealed in 2017 23, Calretinin is a perfect and trustworthy diagnostic aid to histological examination of HD, where claimed sensitivity and specificity are 100%.
Various research have reported that Calretinin is a good marker for displaying HD ganglia. The presence of hypertrophic submucosal nerve bundles is a beneficial positive finding because HD is diagnosed based on the absence of a histological characteristic, namely the ganglion cells 24. many large nerves are usually present in Submucosal nerve hypertrophy, shown in the aganglionic rectal submucosa of a patient with HD cases in (Figure 6).

Figure 6. The immunohistochemical staining method detection of Calretinin in the rectal biopsies of the HD case showed hypertrophied nerve fiber (brown color) and no ganglion cells in the submucosa layers (X10).
According to 27, aberrant nerves and aganglionosis are linked to hypertrophied submucosal nerve trunks. Nine (32.1%) of the 28 patients in their study who lacked ganglionic cells had hypertrophied nerve trunks. The diagnosis can be made without additional testing in a state where no ganglion cells are seen, and overt submucosal nerve hypertrophy is present. Unfortunately, some biopsies lack this unmistakable diagnostic evidence or exhibit aberrant traits that require further investigation. The presence of hypertrophic submucosal nerve bundles is a beneficial positive finding for HD because HD is diagnosed based on the absence of a histological characteristic, namely the GCs24. When GCs are absent, the affected part of the colon cannot contract and relax in a coordinated manner. As a response, the nerve fibers present in the affected part of the colon increase in size and number. This increase, known as hypertrophy, is a compensatory mechanism. The purpose is to circumvent the dysfunctional or missing GCs and stimulate the contraction of the intestinal muscles.
The Hematological Study
Our study is the first to address the complete blood cell (CBC) count in HD and compare HD patients with non-HD patients and control, as shown in (table 3).

Table 3. CBC counts in HD, non-HD and control cases.
The result above showed a significant difference (p < 0.05) in Mean ± SE of WBC total, platelets, eosinophil and monocyte between HD patients, non-HD patients and the control group. Meanwhile, the results of neutrophil, lymphocyte, basophil, RBC, and HGB revealed no significant difference between HD patients, non-HD, and control groups. CBC test is usually obtained to ensure the preoperative hematocrit and platelet count are suitable for surgery 28. In most cases, values were within the reference ranges. Usually, it is a standard or nonspecific finding, but the surgeon specialist completes a blood cell (CBC) count if enterocolitis is suspected. Elevating the white blood cell (WBC) count or a pandemic should raise concern for enterocolitis 29. According to disease stage, patient condition, and complications of HD, our results find Leukocytosis; the current findings generally agree with earlier research that demonstrated a substantial difference in WBC total, the primary blood indication of overall inflammation 30,31. The result also showed a significant difference (p < 0.05) in Mean ± SE of blood eosinophil and monocyte percentage. The multifunctional leukocytes known as eosinophils are crucial to the beginning and controlling inflammation 32, and increases in monocytes indicate that the body is working to combat certain diseases 33. 33. High circulating monocyte levels are thought to facilitate the fast migration of many cells into damaged tissues in response to inflammatory signals 34. Monocytes rapidly undergo phenotypic cell changes as they enter peripheral tissue after migrating from the circulation 35. According to the current finding, HD patients’ platelet counts significantly increased (p > 0.05) compared to a non-HD group. In addition to their critical functions in hemostasis and thrombosis, platelets also play key roles in microbial host defense, wound healing, and angiogenesis 36, and their involvement in inflammatory pathways, which has been shown in several studies, as well as their involvement in inflammatory illnesses. 37 38. The 39 found a cute inflammatory disorders, chronic infections, and inflammatory diseases, such as connective tissue disorders and inflammatory bowel disease, both result in changes in platelet indices. As a consequence of the disease coupled with enterocolitis, failure to diagnose early HD can also result in iron deficiency anemia, hypoproteinemia, and hypoalbuminemia due to protein-losing enteropathy; this may also be the cause of the change in CBC 40. Additionally, viral diseases like rotavirus and cytomegalovirus can cause hematological changes 41 In extremely few instances, intractable anemia may be detected on a patient’s CBC as one of HD’s clinical symptoms 42, consistent with the recent findings. While the results of lymphocytes, neutrophils, basophils, RBC count, HGB, and HCT in the blood did not differ significantly (p > 0.05) between HD patients, non-HD patients, and the control group, it was clear that the disease had no impact on these cells because there was no change in their counts.
Prenatal causes
In a recent study, some worrying maternal risk factors were highlighted during pregnancy were the age of the mother at conception, shown in Table (4), maternal illness, intake of drugs, type of Childbirth, and number of previous maternal abortions, all of them offer a non-significant difference (p>0.05) between the HD group and non-HD group. Still, consanguineous marriage was detected and showed a significant difference (p<0.05) between the HD group and non-HD group shown in table (5).
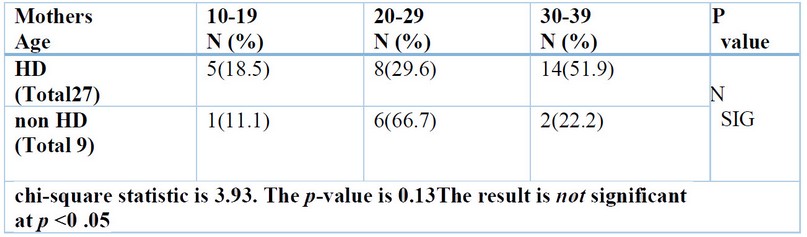
Table 4. Mother’s age of the studied groups.
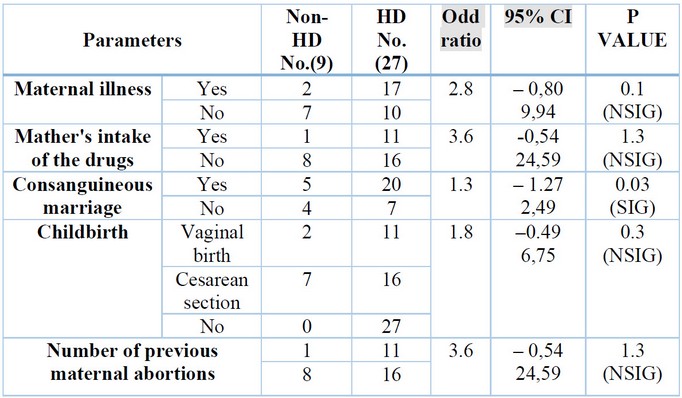
Table 5. Mother’s signs of the studied groups.
The result above found non-significant associations between the mother’s age at Childbirth and the development of HD; this result is consistent with the results of a study by 43. Although not statistically significant, some studies associated maternal age with HD frequency; these studies found HD was more common in infants with mothers aged ≥30 years at Childbirth. However, 44 found no significant association between mothers’ age and HD frequency.
Numerous studies have found that the risk of congenital disabilities rises with maternal age, especially in moms older than 35 years. At the same time, 45 could not discover a connection between congenital abnormalities and maternal age in their investigation. The development of HD is influenced by several risk factors, including Down syndrome (trisomy 21). They anticipated that longer maternal ages would result in a more significant proportion of newborns with HD since pregnant women over the age of 35 have a higher probability of having a baby with trisomy 21 46. Aganglionosis of the colon diagnosed during pregnancy is highly uncommon; just two cases have been documented in the literature, and both of them had polyhydramnios and dilated bowel loops. 47,48.Where the mother-to-be displayed several symptoms related to her prenatal illnesses, such as Polyhydramnios. Apart from noninvasive ultrasound, prenatal diagnosis of HD has been attempted, but with limited success, by measuring amniotic fluid disaccharides activity in the amniotic fluid 49. The study of 50 antenatal ultrasounds of a term male newborn at 33 weeks revealed decreased growth and Polyhydramnios. Following delivery, testing revealed the infant had HD and other abnormalities. Some authors say Polyhydramnios appears to be a little more common (9% in their cohort vs. 1% in the general population). Additionally, the mothers in the current study, in some instances, had Covid-19, Toxoplasmosis, Rubella, Asthma, Anemia, Diabetes and Hypertension, where numerous writers have provided evidence suggesting that viruses may have long-lasting adverse effects on the fetus. High levels of maternal inflammation during viral infection can affect all facets of fetal brain development and result in extensive neurological aftereffects 51,52.
Concerns about spreading the virus and damaging fetuses by vertical transmission 53 exist. According to other investigators, the danger of vertical transmission was minimal, and neither antenatal nor postnatal evaluations revealed any congenital disabilities 54. Panahi 55 findings do not point to a significant increase in the probability of abortion or genetic abnormalities in newborns in COVID-19-infected mothers. According to 56, SARS infection has been linked to a higher incidence of intrauterine development retardation but not SARS-CoV-2 infection. Since the infection is novel, monitoring COVID-19-positive pregnant women is therefore essential to prevent negative maternal and fetal consequences 54. Congenital abnormalities, stillbirth, and increased fetal growth remain the most severe adverse consequences of diabetic pregnancy, according to a prior study 57. Congenital anomalies were described by 58. It is undeniably connected between more significant risks of congenital abnormalities and poor glycemic control at the time of conception and during the first trimester. The 59 investigated the impact of chronic hypertension on fetal growth and concluded that it increased the likelihood of intrauterine growth restriction. No, HD-causing prenatal illness exists. According to the findings of the study 60, 9% and 6% of the population, respectively, had gestational hypertension and diabetes. Similar rates (10% and 4%, respectively) have been recorded for the general population. In their article, 61 describes how maternal asthma during pregnancy affects fetal growth and development, which may impair the future health of the offspring. Although many expectant mothers abstain from taking any medications while they are pregnant, frequent asthma attacks may lower the amount of oxygen in the mother’s blood, which in turn reduces the amount of oxygen in the fetus and causes issues with the fetus’s development. When using the asthma medications as directed.
According to a recent systematic analysis, iron deficiency during the first and second trimesters increases maternal morbidity and the likelihood of unfavorable pregnancy outcomes, such as low birth weight preterm, or intrauterine growth restriction 62. Fetal hazards result from low fetal iron levels, which can be caused by maternal anemia or pregnancy complications that impair the transfer of fetal iron from the mother 63. Several findings state that there is no connection between maternal Hb levels and growth retardation 64. Furthermore, 65 found a link between low maternal Hb levels and unfavorable pregnancy outcomes, including preterm, low birth weight, fetal death, and other medical anomalies. According to the study’s questionnaire, some pregnant mothers did not take any medications other than some vitamins and were under medical supervision. Still, some of them admitted to taking medications for diseases they experienced during pregnancy, some of which were taken under medical supervision, and others were self-medication. Because it is simple to obtain medicines from pharmacies or drug stores without a prescription, time savings, the lengthy wait times for medical services, the perception that their sickness was not severe, and the lower cost of self-medication may also be contributing factors 66. They concluded that taking a medication that is contraindicated during pregnancy does not necessarily represent a high-risk situation; however, other authors supported the idea that taking drugs that are illegal while pregnant poses a risk to both the mother and the fetus because drug exposure during this time is likely to result in congenital malformations 67. Others consumed herbs. There are no studies or studies that are similar to this topic. Still, there are studies on the effects of using herbs indiscriminately while pregnant, which puts the mother and fetus at risk because these herbs contain substances that may be hazardous to the mother and fetus 68, and this may be a valid reason for their children to have many diseases and congenital malformations.
Additionally, the results of the recent study did not discover a connection between congenital disabilities and the kind of pregnancy or prior abortions. This differs from other studies 69,70. Previous research in Iraq has linked prior abortions to congenital abnormalities 71. In our study, from the maternal risk factors, only Consanguinity shows a significant difference (p<0.05) between the HD group and non-HD group. Consanguinity and HD: The prevalence of consanguineous marriages—marriages between close biological relatives—varies greatly worldwide, from less than 1% in North America and most of Europe to over 50% in some areas of Asia and the Middle East. The prevalence of HD in the offspring of consanguineous parents has not been widely recorded in these places. However, Consanguinity between parents is frequent in the Middle East and several nations in Asia 72. According to a study looking at HD in Oman 73, parents of HD patients had a consanguinity rate of 75% compared to 33% in the general community. According to a more recent study from Bangladesh, parents of HD babies had a 16% consanguinity rate compared to a 10% rate in the general community. Twelve percent (15/129) of the families were consanguineous, according to a more recent study from China 74. It is advised to avoid consanguineous marriage because it appears to be a risk factor for HD. It has been shown that Consanguinity increases the likelihood that a husband and wife may have a gene that originated from a common ancestor. Numerous studies have shown that children of such a marriage are more likely to be homozygous for a dangerous gene and subsequently have autosomal recessive genetic diseases 75-77. Other researchers, however, examined seven (25%) children with consanguineous parents who had HD (P = 1.000) and found no evidence of a connection between the two.
Additionally, Consanguinity between parents is quite common in the Middle East, and consanguineous marriage is also quite common there. As a result, a future study that includes cases from all regional communities could be conducted to look into the genetic predisposition of HD and the significance of consanguinity 78. Nevertheless, these publications provide compelling evidence that a maternal risk factor harms fetal growth and pregnancy outcomes. However, referring to some risk variables as potentially dangerous rather than as an adequate appraisal clearly demonstrating a harmful effect on the fetus would be preferable. Furthermore, it is crucial to note that risk factors are frequently linked to other pathologic disorders, making it difficult to determine if a risk factor for the mother directly causes or even contributes to an increase in HD.
CONCLUSION
The current results showed that TB stain can quickly be done for diagnosis of HD; the MCs count in the submucosa is a significant criterion in HD and inflammation. Calretinin immunostaining is a reliable and beneficial test for the diagnostic of HD, aid in histopathological examination of suspected HD, and detect hypertrophic nerve bundles, which may aid in the diagnosis of HD. Submucosal nerve bundle hypertrophy is considered an adjuvant histological criterion for detecting HD. Finally, the result of the CBC count was not related to HD.
Acknowledgments: We thank the Department of Biology, College of Science, Mustansiriyah University (http://uomustansiriyah.edu.iq/), and Baghdad for advice and support.
REFERENCES
1. Klein M, Varga I. Hirschsprung’s disease-recent understanding of embryonic aspects, etiopathogenesis and future treatment avenues. Medicina (Kaunas) Internet. 2020; 56(11):611. Available from: http://dx.doi.org/10.3390/medicina56110611.
2. Gustafson E, Larsson T, Danielson J. Controlled outcome of Hirschsprung’s disease beyond adolescence: a single center experience. Pediatr Surg Int Internet. 2019;35(2):181–5. Available from: http://dx.doi.org/10.1007/s00383-018-4391-5.
3. Holschneider AM, Meier-Ruge W, Ure BM. Hirschsprung’s disease and allied disorders–a review. Eur J Pediatr Surg Internet. 1994;4(5):260–6. Available from: http://dx.doi.org/10.1055/s-2008-1066115.
4. Gorbatyuk OM. Current approaches to diagnosis and treatment of Hirschsprung disease in newborns and infants (literature review and first-hand experience). Wiad Lek Internet. 2022;75(4):1026–30. Available from: http://dx.doi.org/10.36740/wlek20220420120.
5. Bahrami A, Joodi M, Moetamani-Ahmadi M, Maftouh M, Hassanian SM, Ferns GA, et al. Genetic background of hirschsprung disease: A bridge between basic science and clinical application. J Cell Biochem Internet. 2018;119(1):28–33. Available from: http://dx.doi.org/10.1002/jcb.26149.
6. Kapur RP, Ambartsumyan L, Smith C. Are we underdiagnosing hirschsprung disease? Pediatric and Developmental Pathology. Pediatric and Developmental Pathology: The Official Journal of the Society for Pediatric Pathology and the Paediatric Pathology Society Internet. 2020;23(1):60–71. Available from: http://dx.doi.org/10.1177/1093526619889434.
7. Lim KH, Wan WK, Lim TKH, Loh AHL, Nah SA, Chang KTE. Primary diagnosis of Hirschsprung disease-Calretinin immunohistochemistry in rectal suction biopsies, with emphasis on diagnostic pitfalls. World Journal of Pathology. 2014;3(3).
8. Rytting H, Dureau ZJ, Vega JV, Rogers BB, Yin H. Autopsy study of calretinin immunohistochemistry in the anorectal canal in young infants and potential implications for rectal biopsy approach in the neonatal period. Pediatr Dev Pathol Internet. 2021;24(6):542–50. Available from: http://dx.doi.org/10.1177/10935266211030172.
9. Ravanbakhsh N, Kesavan A. The role of mast cells in pediatric gastrointestinal disease. Ann Gastroenterol Internet. 2019;32(4):338–45. Available from: http://dx.doi.org/10.20524/aog.2019.0378.
10. Yasseen HA. Toluidine blue stain and crystal violet stain versus H and E stain in the diagnosis of Hirschsprung’s disease: A study in Sulaimani city in Kurdistan/Iraq. Ann Pathol Lab Med. 2015;2:A54-61.
11. Suvara K. Bancrofts theory and practic of histological techniques. Eight Edtion. Churchill Livingstone Elsevier; 2019.
12. Anuradha GP, Anita A, Seemant SK, Pratima S. t cell profile in appendicitis. Indian Journal of Pathology and Oncology. 2017;4(4):555–9.
13. Kobayashi H, Yamataka A, Fujimoto T, Lane GJ, Miyano T. Mast cells and gut nerve development: implications for Hirschsprung’s disease and intestinal neuronal dysplasia. J Pediatr Surg Internet. 1999;34(4):543–8. Available from: http://dx.doi.org/10.1016/s0022-3468(99)90069-6.
14. Li W-T, Luo Q-Q, Wang B, Chen X, Yan X-J, Qiu H-Y, et al. Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. FASEB J Internet. 2019;33(2):2435–50. Available from: http://dx.doi.org/10.1096/fj.201800935RR.
15. Yadav AK, Mishra K, Mohta A, Agarwal S. Hirschsprung’s disease: Is there a relationship between mast cells and nerve fibers? World J Gastroenterol Internet. 2009;15(12):1493. Available from: http://dx.doi.org/10.3748/wjg.15.1493.
16. Demirbilek S, Ozardali HI, Aydm G. Mast-cells distribution and colonic mucin composition in Hirschsprung’s disease and intestinal neuronal dysplasia. Pediatr Surg Int Internet. 2001;17(2–3):136–9. Available from: http://dx.doi.org/10.1007/s003830000467.
17. Hermanowicz A, Debek W, Dzienis-Koronkiewicz E, Chyczewski L. Topography and morphometry of intestinal mast cells in children with Hirschsprung’s disease. Folia Histochem Cytobiol Internet. 2008;46(1):65–8. Available from: http://dx.doi.org/10.2478/v10042-008-0008-5.
18. Do Carmo Neto JR, Braga YLL, Da Costa AWF, Lucio FH, Do Nascimento TC, Reis MA, et al. Biomarkers and their possible functions in the intestinal microenvironment of chagasic megacolon: an overview of the (neuro) inflammatory process. Journal of Immunology Research. 2021.
19. Nanagas VC, Kovalszki A. Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: A comprehensive review. Clin Rev Allergy Immunol Internet. 2019;57(2):194–212. Available from: http://dx.doi.org/10.1007/s12016-018-8695-y
20. Kini U. Pathology of the gut motility disorders: Hirschsprung’s disease. In: Surgical Pathology of the Gastrointestinal System. Singapore: Springer Singapore; 2022. p. 339–74.
21. Singh SK, Rajoria K. Ayurvedic management of chronic constipation in Hirschsprung disease-A case study. Journal of Ayurveda and Integrative Medicine. 2018;9(2):131–5.
22. Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol Internet. 2018; Available from: http://dx.doi.org/10.1016/j.jaci.2018.01.034.
23. Musa ZA, Qasim BJ, Ghazi HF, Al Shaikhly AWAK. Diagnostic roles of Calretinin in hirschsprung disease: A comparison to neuron-specific enolase. Saudi J Gastroenterol Internet. 2017;23(1):60–6. Available from: http://dx.doi.org/10.4103/1319-3767.199118.
24. Green N, Smith CA, Bradford MC, Ambartsumyan L, Kapur RP. Rectal suction biopsy versus incisional rectal biopsy in the diagnosis of Hirschsprung disease. Pediatr Surg Int Internet. 2022;38(12):1989–96. Available from: http://dx.doi.org/10.1007/s00383-022-05246-4.
25. Naimi A, Shegeft E. Evaluation of the Diagnostic Value of Calretinin Immunohistochemistry Assay in the Superficial Rectal Biopsy of Children Suspected of Hirschsprung’s Disease. Iranian Journal of Neonatology. 2022;13(1).
26. Barshack I, Fridman E, Goldberg I, Chowers Y, Kopolovic J. The loss of calretinin expression indicates aganglionosis in Hirschsprung’s disease. J Clin Pathol Internet. 2004;57(7):712–6. Available from: http://dx.doi.org/10.1136/jcp.2004.016030.
27. Alizai NK, Batcup G, Dixon MF, Stringer MD. Rectal biopsy for Hirschsprung’s disease: what is the optimum method? Pediatr Surg Int Internet. 1998;13(2–3):121–4. Available from: http://dx.doi.org/10.1007/s003830050264.
28. Umesh G, Bhaskar SB, Harsoor SS, Dongare PA, Garg R, Kannan S, et al. Preoperative investigations: Practice guidelines from the Indian society of anaesthesiologists. Indian J Anaesth Internet. 2022;66(5):319–43. Available from: http://dx.doi.org/10.4103/ija.ija_335_22.
29. Frykman PK, Kim S, Wester T, Nordenskjöld A, Kawaguchi A, Hui TT. & HAEC Collaborative Research Group. Critical evaluation of the Hirschsprungassociated enterocolitis (HAEC) score: a multicenter study of 116 children with Hirschsprung disease. Journal of pediatric surgery. 2018;53(4):708–17.
30. Alkarzae M, Alsanosi A, Alharbi M, Altamimi F, Alzendi N. Role of infection in post-tonsillectomy secondary haemorrhage-an experience at King Abdulaziz University Hospital. Glob J Otolaryngol. 2017;6.
31. Lefta AS, Daway HG, Jouda J. Red Blood Cells detecting depending on binary conversion at multi threshold values. Al-Mustansiriyah J Sci Internet. 2022;33(1):69–76. Available from: http://dx.doi.org/10.23851/mjs.v33i1.1079.
32. Diny NL, Rose NR, Čiháková D. Eosinophils in autoimmune diseases. Front Immunol Internet. 2017; 8:484. Available from: http://dx.doi.org/10.3389/fimmu.2017.00484.
33. Lichtman MA, Beatler E, Kipps TJ, Seligsohn U, Prchal JT. Williams Hematology. The McGrawHill Companies. 2010;
34. Arol C, Yona S. Origins and tissuecontext dependent fates of blood monocytes. Immunology and cell biology. 2009;87(1):30–8.
35. Al-Sarray ZA, Hussein RH, Al-Hafidh AH, Al-Rayahi IA. Vitamin D deficiency associates with disease severity in rheumatoid arthritis patients. Al-Mustansiriyah J Sci Internet. 2023;33(5):33–8. Available from: http://dx.doi.org/10.23851/mjs.v33i5.1310.
36. Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev Internet. 2015;29(3):153–62. Available from: http://dx.doi.org/10.1016/j.blre.2014.10.003.
37. Kostakis ID, Angelidou M, Kambouri K, Gardikis S, Cholidou GK, Gioka T, et al. Hematological diagnostic markers of acute appendicitis in children. Hell Cheirourgike Internet. 2018;90(3):127–36. Available from: http://dx.doi.org/10.1007/s13126-018-0457-z.
38. Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci Internet. 2010;67(4):499–511. Available from: http://dx.doi.org/10.1007/s00018-009-0205-1.
39. Schafer AI. Thrombocytosis and thrombocythemia. Blood Rev Internet. 2001;15(4):159–66. Available from: http://dx.doi.org/10.1054/blre.2001.0162.
40. Sun X, Chu J, Li C, Deng Z. Hirschsprung’s disease presenting as intractable anemia: a report of two cases and review of the literature. BMC Pediatr Internet. 2020;20(1):525. Available from: http://dx.doi.org/10.1186/s12887-020-02423-z
41. Urrechaga E, Aguirre U, España PP, De Guadiana LG. Complete blood counts and cell population data from Sysmex XN analyser in the detection of SARSCoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM). 2021;59(2):e57–60.
42. Al-Shamaileh T, Hashem H, Farhoud E, Al-Edwan A, Alomari MS, Levitt MA. MDelayed diagnosis of Hirschsprung disease presenting initially as anemia: A case report. Journal of Pediatric Surgery Case Reports. 2023.
43. Sukarelawanto AVR, Ritana A, Balela N, Putri WJK, Sirait DN, Paramita VMW. & Makhmudi, A. Postoperative enterocolitis assessment using two different cut-off values in the HAEC score in Hirschsprung patients undergoing Duhamel and Soave pull-through. BMC pediatrics. 2020;20(1):1–6.
44. Granström L, Svenningsson A, Hagel A. Maternal risk factors and perinatal characteristics for Hirschsprung disease. Pediatrics. 2016;138.
45. Ajao AE, Adeoye IA. Prevalence, risk factors and outcome of congenital anomalies among neonatal admissions in OGBOMOSO, Nigeria. BMC Pediatr Internet. 2019;19(1):88. Available from: http://dx.doi.org/10.1186/s12887-019-1471-1.
46. Demehri FR, Halaweish IF, Coran AG. Hirschsprungassociated enterocolitis: pathogenesis, treatment and prevention. Pediatr Surg Int. 2013; 29:873–81.
47. Wrobleski D, Wesselhoeft C. Ultrasonic diagnosis of prenatal intestinal obstruction. J Pediatr Surg Internet. 1979;14(5):598–600. Available from: http://dx.doi.org/10.1016/s0022-3468(79)80146-3.
48. Vermesh M, Mayden KL, Confino E, Giglia RV, Gleicher N. Prenatal sonographic diagnosis of Hirschsprung’s disease. J Ultrasound Med Internet. 1986;5(1):37–9. Available from: http://dx.doi.org/10.7863/jum.1986.5.1.37.
49. Aldaffaa M, Mahfouz A, Alaqeel S, Alakeel HA, Al Naamshan M. Hirschsprung’s disease in a genetically diagnosed Cri-du-chat syndrome baby. J Pediatr Surg Case Rep Internet. 2023;91(102600):102600. Available from: http://dx.doi.org/10.1016/j.epsc.2023.102600.
50. Broch A, Trang H, Montalva L, Berrebi D, Dauger S, Bonnard A. Congenital central hypoventilation syndrome and Hirschsprung disease: A retrospective review of the French National Registry Center on 33 cases. J Pediatr Surg Internet. 2019;54(11):2325–30. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2019.02.014.
51. Cornish EF, Filipovic I, Åsenius F, Williams DJ, McDonnell T. Innate immune responses to acute viral infection during pregnancy. Front Immunol Internet. 2020; 11:572567. Available from: http://dx.doi.org/10.3389/fimmu.2020.572567.
52. Leung KKY, Hon KL, Yeung A, Leung AKC, Man E. Congenital infections in Hong Kong: an overview of TORCH. Hong Kong Med J Internet. 2020;26(2):127–38. Available from: http://dx.doi.org/10.12809/hkmj198287.
53. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord Internet. 2020; 277:5–13. Available from: http://dx.doi.org/10.1016/j.jad.2020.07.126.
54. Mascio D, Sen C, Saccone G, Galindo A, Grünebaum A, Yoshimatsu J, et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by Coronavirus disease (COVID-19): a secondary analysis of the WAPM study on COVID19. Journal of perinatal medicine. 2019;48(9):950–8.
55. Panahi L, Amiri M, Pouy S. Risks of novel Coronavirus disease (COVID-19) in pregnancy; A narrative review. Arch Acad Emerg Med. 2020;8(1):e34.
56. Salem D, Katranji F, Bakdash T. COVID-19 infection in pregnant women: Review of maternal and fetal outcomes. Int J Gynaecol Obstet Internet. 2021;152(3):291–8. Available from: http://dx.doi.org/10.1002/ijgo.13533.
57. Schaefer-Graf U, the Diabetic Pregnancy Study Group, Napoli A, Nolan CJ. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia Internet. 2018; Available from: http://dx.doi.org/10.1007/s00125-018-4545-y.
58. Zabihi S, Loeken MR. Understanding diabetic teratogenesis: where are we now and where are we going? Molecular Causes of Diabetic Teratogenesis. Birth Defects Res A Clin Mol Teratol Internet. 2010;88(10):779–90. Available from: http://dx.doi.org/10.1002/bdra.20704.
59. Haelterman E, Breart G, Paris-Liado J, Dramaix M, Tchobrousky C. Effect of uncomplicated chronic hypertension on the risk of smallforgestational age birth. Am J Epidemiol. 1997;145:689–95.
60. Broch A, Trang H, Montalva L, Berrebi D, Dauger S, Bonnard A. Congenital central hypoventilation syndrome and Hirschsprung disease: A retrospective review of the French National Registry Center on 33 cases. J Pediatr Surg Internet. 2019;54(11):2325–30. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2019.02.014.
61. Meakin AS, Saif Z, Seedat N, Clifton VL. The impact of maternal asthma during pregnancy on fetal growth and development: a review. Expert Rev Respir Med Internet. 2020;14(12):1207–16. Available from:http://dx.doi.org/10.1080/17476348.2020.1814148
62. Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O’Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutr Internet. 2018;148(11):1716–22. Available from: http://dx.doi.org/10.1093/jn/nxy151.
63. Monk C, Georgieff MK, Xu D, Hao X, Bansal R, Gustafsson H, et al. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatr Res Internet. 2016;79(3):482–8. Available from: http://dx.doi.org/10.1038/pr.2015.248.
64. Kaltreider DF, Kohl S. Epidemiology of preterm delivery. Clin Obstet Gynecol Internet. 1980;23(1):17–31. Available from: http://dx.doi.org/10.1097/00003081-198003000-00005.
65. Garn SM, Ridella SA, Petzold AS, Falkner F. Maternal hematologic levels and pregnancy outcomes. Semin Perinatol. 1981;5(2):155–62.
66. Hoeltzenbein M, Slimi S, Fietz A-K, Stegherr R, Onken M, Beyersmann J, et al. Increasing use of newer antiseizure medication during pregnancy: An observational study with special focus on lacosamide. Seizure Internet. 2023; 107:107–13. Available from: http://dx.doi.org/10.1016/j.seizure.2023.02.015.
67. Black RA, Hill DA. Over-the-counter medications in pregnancy. Am Fam Physician. 2003;67(12):2517–24.
68. Bruno LO, Simoes RS, de Jesus Simoes M, Girão MJBC, Grundmann O. Pregnancy and herbal medicines: An unnecessary risk for women’s health-A narrative review. Phytother Res Internet. 2018;32(5):796–810. Available from: http://dx.doi.org/10.1002/ptr.6020.
69. Ronya R, Gupta D, Ghosh SK, Narang R, Jain KB. Spectrum of congenital surgical malformations in newborns. J Indian Med Assoc. 2002;100(9):565–6.
70. Cherian AG, Jamkhandi D, George K, Bose A, Prasad J, Minz S. Prevalence of congenital anomalies in a secondary care hospital in South India: A cross-sectional study. J Trop Pediatr Internet. 2016;62(5):361–7. Available from: http://dx.doi.org/10.1093/tropej/fmw019.
71. Ameen SK, Alalaf SK, Shabila NP. Pattern of congenital anomalies at birth and their correlations with maternal characteristics in the maternity teaching hospital, Erbil city, Iraq. BMC Pregnancy Childbirth Internet. 2018;18(1):501. Available from: http://dx.doi.org/10.1186/s12884-018-2141-2.
72. Puri P, Nakamura H. Familial Hirschsprung’s Disease. In: Hirschsprung’s Disease and Allied Disorders. Cham: Springer International Publishing; 2019. p. 115–9.
73. Rajab A, Freeman NV, Patton MA. Hirschsprung’s disease in Oman. J Pediatr Surg Internet. 1997;32(5):724–7. Available from: http://dx.doi.org/10.1016/s0022-3468(97)90015-4.
74. Xiao J, Hao L-W, Wang J, Yu X-S, You J-Y, Li Z-J, et al. Comprehensive characterization of the genetic landscape of familial Hirschsprung’s disease. World J
75. Mobarak AM, Chaudhry T, Brown J, Zelenska T, Khan MN, Chaudry S, et al. Estimating the health and socioeconomic effects of cousin marriage in south Asia. J Biosoc Sci Internet. 2019;51(3):418–35. Available from: http://dx.doi.org/10.1017/S0021932018000275.
76. Al-Hamed MH, Alsahan N, Tulbah M, Kurdi W, Ali W, Sayer JA, et al. Fetal anomalies associated with novel pathogenic variants in TMEM94. Genes (Basel) Internet. 2020;11(9):967. Available from: http://dx.doi.org/10.3390/genes11090967.
77. Najafi K, Mehrjoo Z, Ardalani F, Ghaderi-Sohi S, Kariminejad A, Kariminejad R, et al. Identifying the causes of recurrent pregnancy loss in consanguineous couples using whole exome sequencing on the products of miscarriage with no chromosomal abnormalities. Sci Rep Internet. 2021;11(1):6952. Available from: http://dx.doi.org/10.1038/s41598-021-86309-9.
78. Al-Taher R, Daradkeh HT, Hadadin H, Obiedat A, Hijazein Y, Hijazein L, et al. Children with Hirschsprung disease in a developing country: A cohort study of the predictors of a positive rectal biopsy result. Medicine (Baltimore) Internet. 2022;101(46):e31601. Available from: http://dx.doi.org/10.1097/MD.0000000000031601.
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation: Abdul Hussein R A., H. AL-Sharqi S A, Mehdi N K., Joda A E. Evaluation of Calretinin and enumeration of mast cells in rectum tissue biopsies of Hirschsprung and non-Hirschsprung disease in neonate and infant. Revis Bionatura 2023;8 (4) 32. http://dx.doi.org/10.21931/RB/2023.08.04.32
Additional information Correspondence should be addressed to rusul_abass@yahoo.com



















