Vol 1 No 4 2016 – 7
ARTÍCULO DE REVISIÓN
Microalgae: An outstanding tool in nanotechnology
Microalgas: Una excelente herramienta en nanotecnología
Available from: http://dx.doi.org/10.21931/RB/2016.01.04.7
Si Amar Dahoumane,1* Mourad Mechouet,2 Francisco J. Alvarez,1 Spiros N. Agathos,1 Clayton Jeffryes.3*
_______________________________________________________________________________________________________________________
Abstract
Microalgae are microorganisms of choice in biotechnology thanks to their wide range of potential bio-applications, such as over-expression of pigments, bioremediation, biofuel production and toxicity studies. Recently, microalgae have been gaining attention from material scientists worldwide owing to their versatility, and the ease and the variety of procedures through which the biosynthesis of valuable nanomaterials is implemented. This has resulted mainly in the production of nanoparticles made of noble metals, alloys, oxides and chalcogenides. Although still burgeoning, the biosynthesis of nanomaterials based on the exploitation of microalgal resources may thrive and witness dramatic developments in the near future.
Keywords: Microalgae, Nanobiotechnology, Biosynthesis, Nanomaterials, Sustainability, Photobioreactors.
_______________________________________________________________________________________________________________________
Resumen
Las micro algas son organismos de elección en la biotecnología gracias a su amplia gama de potenciales bio-aplicaciones, como la sobre expresión de pigmentos, la biorremediación, la producción de biocombustibles y los estudios de toxicidad. Recientemente, las micro algas han estado ganando atención, a nivel mundial, de los científicos de materiales debido a su versatilidad, facilidad y la variedad de procedimientos a través de los cuales se implementa la biosíntesis de valiosos nanos materiales. Esto ha funcionado principalmente en la producción de nano partículas hechas de metales nobles, aleaciones, óxidos y halogenuros. Aunque sigue floreciendo, la biosíntesis de nano materiales basada en la explotación de las micro alga estos procedimientos pueden prosperar y ser testigo de dramáticos acontecimientos en un futuro próximo.
Palabras clave: Microalgas, Nanobiotecnología, Biosíntesis, Nanomateriales, Sustentabilidad, Fotobioreactores.
_______________________________________________________________________________________________________________________
Abstract
Microalgae are microorganisms of choice in biotechnology thanks to their wide range of potential bio-applications, such as over-expression of pigments, bioremediation, biofuel production and toxicity studies. Recently, microalgae have been gaining attention from material scientists worldwide owing to their versatility, and the ease and the variety of procedures through which the biosynthesis of valuable nanomaterials is implemented. This has resulted mainly in the production of nanoparticles made of noble metals, alloys, oxides and chalcogenides. Although still burgeoning, the biosynthesis of nanomaterials based on the exploitation of microalgal resources may thrive and witness dramatic developments in the near future.
Keywords: Microalgae, Nanobiotechnology, Biosynthesis, Nanomaterials, Sustainability, Photobioreactors.
_______________________________________________________________________________________________________________________
Introduction
Microalgae are generally single-celled, colonial or filamentous photosynthetic microorganisms belonging to several algal divisions, such as Chlorophyta, Charophyta and Bacillariophyta, and Kingdoms, such as Plantae, Chromista and Protozoa. Microalgae, except for the prokaryotic cyanobacteria, have a nucleus surrounded by a membrane and are therefore eukaryotes. Microalgae form a substantial part of the World’s biodiversity. By some estimates, there are hundreds of thousands to millions of microalgal species, most of which have not been isolated or characterized.1, 2 Microalgae display a wide variety of shapes and sizes and can be found in different habitats, either aquatic or semiaquatic, such as seas and lakes, rivers and swamps, polar areas and deserts.
Microalgae possess the ability to convert solar energy and CO2 into high levels of lipids and secondary carotenoids in their biomass. This synthesis is carried out at ambient temperatures and pressures and in simple aqueous media at benign pH values. As a result, microalgae are emerging as important, cost-effective cell factories for the production of commercial products such as carotenoids3, 4 and fatty acids.5 The conversion of algal lipids into biofuels is also a promising area of research.6 Recently, the United States Department of Energy (2016) launched the MEGA-BIO program to investigate algae as a biofuel platform. To this end, several species of microalgae have already demonstrated the capacity to accumulate intracellular concentrations of over 50% lipids on a dry cell weight basis under species-specific stresses such as nitrate7 or silicon8 limitation, while the carotenoid producer Haematococcus pluvialis has the ability to accumulate up to 5% of its biomass as astaxanthin, a valuable carotenoid.9 Recent efforts have also used genetic engineering to enable the production of recombinant proteins,10 even inducing gene expression by optogenetic control.11
A nanoparticle (NP) is an object with at least one dimension spanning from 1 nm to 200 nm. Several methodologies have been designed in order to produce inorganic nanomaterials of different compositions, shapes and sizes thus enabling the nanostructures to display interesting and valuable properties. Besides the physico-chemical routes,12, 13 biological processes have proved efficient in the biosynthesis of a large range of nanomaterials, such as oxides, chalcogenides and metallic NPs, using several resources, namely bacteria,14 fungi,15 plants16 and algae.17, 18
The aim of the present review is to provide the readership with some interesting facts about the exploitation of microalgae in the emerging field of inorganic nanoparticle synthesis using biological resources in which algae, in general, and microalgae, in particular, are gaining much more attention owing to their diversity, availability and physiological features.
Production of metallic nanoparticles using microalgae
Among the community of nanomaterial scientists, several methodologies have been devised in order to implement the synthesis of metallic nanoparticles (Me-NPs) using microalgae starting from aqueous solutions of the corresponding salts. Authors tend to classify these methodologies according to arbitrary criteria. As the field is quite new, however, attracting new teams of scientists worldwide, this aspect is yet to be clarified. So far, researchers describe their findings as “intracellular” when the biosynthesis of the NPs occurs inside the cells and “extracellular” for the other occurrences even if their experimental setups do not involve cells directly but extracted biomass or biomolecules. In our opinion, the term “extracellular” may be used exclusively when whole, intact cells are involved for processes occurring outside them. If we refer strictly to the process itself, the biosynthesis of nanomaterials based on microalgae abilities can be categorized into four routes (Fig. 1): the first method resides in the exploitation of the extracted biomolecules, either identified and characterized or not, from disrupted cells of microalgae. This method was described within the first papers reporting on the use of microalgae in the field of nanobiotechnology; the second method consists in the use of supernatants devoid of cells, made of culture media from which the cells were removed but without being subjected to any treatment other than their recovery using centrifugation or filtration; the third method relies on harvested whole cells of microalgae, removed from their culture media and re-suspended in distilled water, to promote the biosynthesis of NPs of different natures; the last method relies on the use of living cells of microalgae maintained under their normal culturing conditions.
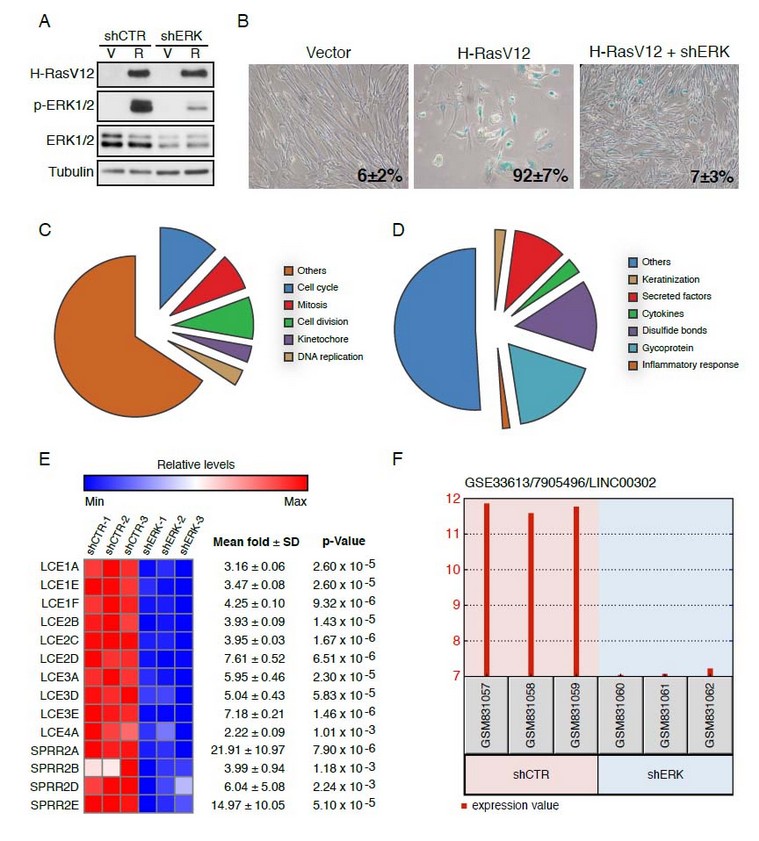
Fig. 1: Different methodologies devised for the exploitation of microalgae in the biosynthesis of nanomaterials.
Biosynthesis of NPs using extracted biomolecules from microalgae
In 2007, a Singaporean team extracted biomolecules from Chlorella vulgaris, a unicellular green microalga, in order to carry out the synthesis of Au-NPs19 and Ag-NPs20 via two distinct methodologies. To obtain gold nanoplates, the biomass of C. vulgaris, first lyophilized, was then subjected to reverse-phase high-performance liquid chromatography (RP-HPLC) until the protein responsible for directing the shape of the NPs, coined GSP for Gold Shape-directing Protein, was isolated.19 Depending on the amount of GSP brought into contact with an aqueous solution of 1 mM HAuCl4, the reaction yields the production of mostly gold nanoplates in the shape of triangles, truncated triangles and hexagons, and a few spherical gold nanoparticles. In the case of Ag-NPs, low and high molecular weight proteins, PLW and PHW, respectively, contained in the biomass of C. vulgaris, were isolated using a dialysis membrane and tested for their ability to promote the reduction of cationic silver into its metallic counterpart.20 As a result, only PHW were able to promote the biosynthesis of Ag-NPs, under the shape of spheres, nanodisks and triangular nanoplates, once challenged by an aqueous solution of AgNO3 (Fig. 2-A).
The pigment C-phycocyanin, either lab-extracted from Limnothrix sp. or commercially purchase and isolated from Spirulina, has been shown to promote the biosynthesis of Ag-NPs starting from an aqueous solution of AgNO3.21 Importantly, the reduction of cationic silver into its metallic counterpart occurs only under light, suggesting that this process is light-dependent. A similar trend has been observed when exopolysaccharides of the green microalga Scenedesmus sp. were used to implement the same synthesis.21
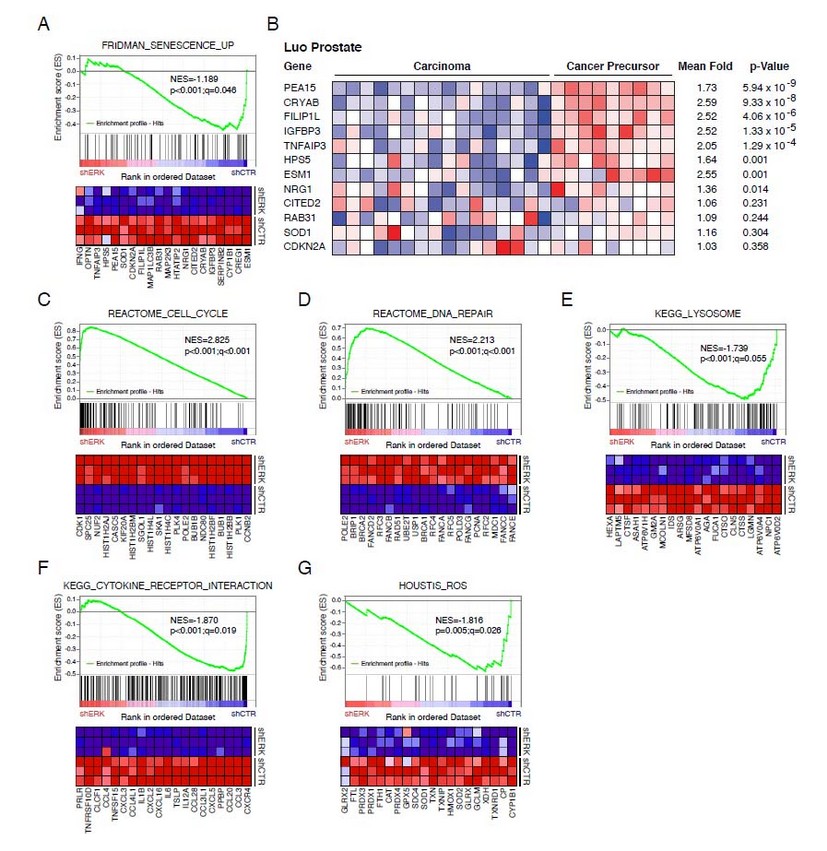
Fig. 2: (A) TEM micrograph of Ag-NPs synthesized by PHW (Proteins of High molecular Weight) extracted from Chlorella vulgaris. Adapted with permission from Ref.20 Copyright (2007) American Chemical Society. (B) TEM micrograph of irregular Ag-NPs produced by Amphora sp. extract. Adapted with permission from Ref.23 Copyright (2015) Elsevier.
Biosynthesis of Me-NPs using cell-free supernatants
Patel and colleagues reported on the exploitation of cell-free supernatants of several species of cyanobacteria and Chlorophyta for the biosynthesis of Ag-NPs starting from an aqueous solution of AgNO3.24 Although the authors did not provide information regarding the NP features nor the nature of the biomolecules involved, the production of the Ag-NPs occurred only when the reaction media were exposed to light. This fact may suggest that the bio-chemical pathways leading to the appearance of Ag-NPs is light-driven. Besides the ability of harvested whole cells, Sudha et al. described the biosynthesis of Ag-NPs of 40-80 nm using the filtrate of the cyanobacterium Microcoleus sp.26
Biosynthesis of Me-NPs using harvested whole cells of microalgae
This method, consisting in the use of harvested whole cells of microalgae for the biosynthesis of Me-NPs, is much easier to implement than the previous ones as the grown cells are harvested through centrifugation/washing cycles before being brought into contact with metallic salts. Even if the cells remain intact and do not suffer from any damage, they may lose their metabolic activity within hours as they are isolated from their growth media and re-suspended in distilled water, the latter medium constituting a stressful environment exposing them to high osmotic pressure.
For instance, whole cells of Plectonema boryanum, a filamentous cyanobacterium, proved efficient in promoting the production of Au-NPs,28 Ag-NPs,29 Pt-NPs30 and Pd-NPs31 when exposed to the corresponding salts of these metals. The experiments were carried out at different temperatures for a certain amount of time. This yielded the appearance of a population of NPs displaying different shapes and different sizes. Importantly, the process occurs both on the surface of the cells and in solution (Fig. 3-A and B, respectively). However, in the absence of thin sections on the cells, it is impossible to either affirm that the process is partly intracellular or to deny it. Other species of cyanobacteria were successfully screened for their ability to promote the production of Ag-NPs24, 32-35 and Au-NPs.36-38 Likewise, several species of green microalgae demonstrated the same capabilities and promoted the production of Ag-NPs24, 25, 39 and Au-NPs.40, 41 Furthermore, harvested cells of Euglena gracilis and Euglena intermedia (Euglenozoa) carried out the biosynthesis of Ag-NPs22 while those of Navicula minima (diatom) were used for the production of Au-NPs.36 The studies that generated photonic pictures of the cells allow to affirm that the NP biosynthesis process is intracellular, independently of the targeted metal and the division of algae to which the species belongs, in a similar way to that reported for living cultures (vide infra).
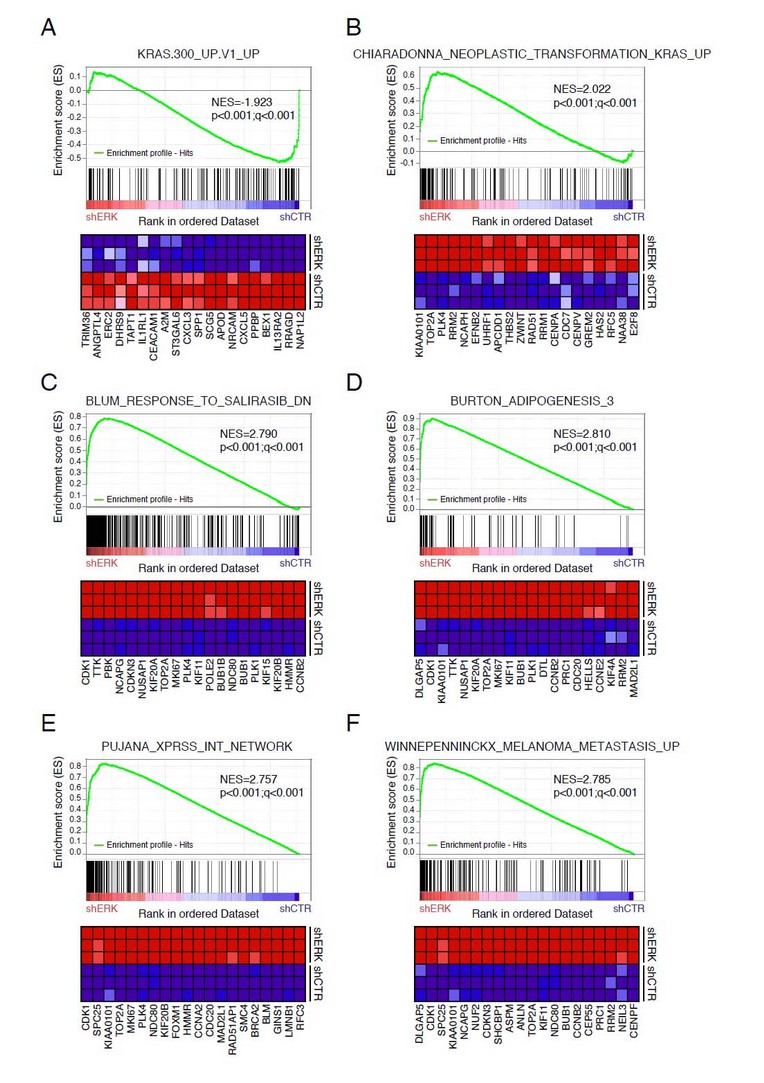
Fig. 3: (A) TEM micrograph of a cell of Plectonema boryanum (cyanobacteria) incubated with an aqueous solution of PdCl2 at 60 °C for 28 days and displaying Pd-NPs on its surface. (B) Pd-NPs in solution. Scale bars: 20 and 200 nm, respectively. Reprinted with permission from Ref.31 Copyright (2007) American Chemical Society.
Biosynthesis of Me-NPs using microalgal living cultures
Among the microorganisms and other biological resources exploited so far, microalgae are the only entities that allow the design of photobioreactors for the sustainable and scalable production of nanomaterials as the experiments are carried out at room temperature and atmospheric pressure, through a one-step process consisting in adding the aqueous solution of the corresponding salts directly to the cells maintained under their usual culturing conditions. On a fundamental point of view, these experiments may also allow to gain insights in the understanding of the underlying mechanisms that govern those processes while the cells are still alive and behave as whole biological entities.17, 42 Since the advent of this methodology a decade ago, cells belonging to more than a half-dozen of algal phyla have been tested for their ability to promote the production of Me-NPs. For instance, the cyanobacterial strains of Anabaena flos-aquae, Calothrix pulvinata and Leptolyngbya foveolarum, were able to implement the biosynthesis of nanoparticles of Au, Ag, Pt and Pd.43 Once synthesized within the cells, the NPs are released into the culture media where they form stable colloids easing therefore their recovery.
The biosynthesis of Ag-NPs was reported using several species of microalgae belonging to cyanobacteria,35 Haptophyta,44 Ochrophyta45 and Chlorophyta44-46 while the biosynthesis of Au-NPs was described using living cultures of Bacillariophyta, cyanobacteria,47, 48 Euglenozoa,49 Chlorophyta46, 50, 51 and Charophyta.52 Moreover, the biosynthesis of bimetallic alloy NPs of silver and gold was implemented using microalgal strains of cyanobacteria53, 54 and Chlorophyta.46
The biosynthesis of noble metal nanomaterials using living cultures of microalgae possesses very special and valuable features. The whole process is depicted in Fig. 4. Each cell – birthplace of the NPs owing to the presence of a complex photosynthetic enzymatic machine – seems to behave as a microscopic photosynthetic factory while the whole culture acts as a photobioreactor. As the biosynthesis process occurs within the cells, the NPs are, in their vast majority, spheres of a few nm in diameter with a narrow distribution in size.47 On their path towards their release to the surrounding medium of the cells, they are wrapped within an organic matrix made of polysaccharides present within and on the cell surface.51, 55 Once released into the supernatant, the NPs form stable colloids due to their capping biopolymers. These latter hinder the NPs from undergoing any alteration in shape and/or size. As a result, the colloids of the emergent NPs can be easily separated from the cells. The addition of fresh culture media will trigger the remaining still viable cells to multiply and allow a new cycle of biosynthesis to be carried out. Bearing in mind the fact that the cells can survive the toxicity of the metallic cations, they can also develop a resistance towards these same cations through mechanisms yet to be clarified, allowing them to handle higher concentrations of those chemicals.52 Another key-feature of microalgae resides in the uptake of silver and gold salts without any predilection, leading thus to the formation of bimetallic alloy NPs of well controlled compositions paving the way to very interesting future developments in the production of NPs of desired compositions.46, 53 All these features make microalgae a biological resource of choice regarding the biosynthesis of nanomaterials. In addition to that, microalgae maintain their NP biosynthetic capabilities when entrapped within either an inorganic matrix56 or organic capsules.57

Fig. 4: The hypothetical mechanism of the biosynthesis of Au-NPs by living cultures of microalgae. Reprinted with permission from Ref.51 Copyright (2014) Springer. First, gold cations are added into a fresh, healthy culture of microalgae. The salts are then internalized by the cells where they are reduced into metallic gold within the thylakoids (Th) through an enzymatic mechanism leading therefore to the formation of Au-NPs. Then, these NPs diffuse to the cell wall (CW) where they are capped by exopolysaccharides (EPS). Finally, the as-produced biohybrids (NPs-EPS) are released into culture media (CM) resulting in the formation of stable colloids.
Biosynthesis of oxide- and chalcogenide-based nanoparticles using microalgae
Compared to the biosynthesis of noble metal NPs, only a handful of papers describe the biosynthesis of oxide- and chalcogenide-based nanomaterials using microalgae. For instance, living cultures of Limnothrix planctonica, Synechoccus leopoldiensis and Pseudoanabaena limnetica (cyanobacteria), proved efficient in removing mercury from their environment by adsorbing Hg2+ cations and, then, transforming them mostly to meta-cinnabar (β-HgS).58 The phytochelatins (PCs) of the marine diatom Phaeodactylum tricornotum were able to promote the fabrication of CdS-NPs through a two-step process: first, the PCs reacted with Cd2+, resulting in the formation of PC-Cd complexes, which were then exposed to Na2S leading to the fabrication of CdS-NPs. A similar process carried out with the pigment C-phycoerythrin, extracted from the cyanobacterium Phormidium tenue, yielded the production of CdS-NPs.59 Harvested whole cells of Spirulina sp. (cyanobacterium) challenged with cadmium nitrate produced photoluminescent CdS-NPs displaying a photocatalytic activity.60
So far, the biosynthesis of iron-based nanomaterials has been implemented through the use of whole cells, either maintained in their culture media or removed, washed and re-suspended in distilled water. The first route involved the use of Anabaena flos-aquae and Calothrix pulvinata (cyanobacteria),61, 62 and Klebsormidium flaccidum (Charophyta) and yielded the apparition of nanorods of akaganeite (FeOOH).62 On the other hand, the use of Euglena gracilis (Euglenozoa) induced the production of tiny spherical NPs of ferrihydrite.63 Through the second route, cells of Chlorococcum sp.64 (Chlorophyta) promoted the production of spherical iron NPs of 20-50 nm while those of Chlorella sp.65 (Chlorophyta) ensured the bioformation of spherical NPs of metallic iron and iron oxide of 5-50 nm in size.
Conclusion and perspectives
The present article reviews the biosynthesis of different nanomaterials using microalgae. It details the particular methodologies devised by materials scientists in concert with biologists and bioengineers resulting in the bioproduction of valuable nanomaterials. It also describes some important technical features regarding each process, opening the route towards the transformation of this technology from an empirical one to one based on well-understood principles and controlled parameters. It appears that microalgae, owing to their diversity and physiology, and the versatility of the designed processes, hold a special position within nanobiotechnology as not only do they possess the features of other microorganisms but they offer additional interesting advantages. This is why this field is expected to evolve tremendously in the near future. Indeed, microalgae offer different ways for their exploitation in the biosynthesis of nanomaterials either at the molecular scale or cellular level. Therefore, we expect the number of released papers in that field to increase and the described nanomaterials to diversify by, for instance, including more oxide- and chalcogenide-based ones, in addition to new metallic-based NPs.
References
1. F. B. J. Metting, J. Ind. Microbiol., 1996, 17, 477-489.
2. J. Sheehan, T. Dunahay, J. Benemann and P. Roessler, A look back at the U.S. Department of Energy’s aquatic species program—biodiesel from algae, National Renewable Energy Laboratory, Golden, CO, USA, 1998.
3. M. A. Borowitzka, J. Appl. Phycol., 2013, 25, 743-756.
4. M. A. Borowitzka, in Single Cell Oils, eds. C. Ratledge and Z. Cohen, AOCS Publishing, Urbana, IL, USA, 2010, pp. 225-240.
5. A. Mendes, A. Reis, R. Vasconcelos, P. Guerra and T. Lopes da Silva, J. Appl. Phycol., 2008, 21, 199-214.
6. M. A. Borowitzka and N. R. Moheimani, Algae for Biofuels and Energy, Springer, New York, NY, USA, 2013.
7. L. Gouveia and A. C. Oliveira, J. Ind. Microbiol. Biotechnol., 2009, 36, 269-274.
8. C. Jeffryes, J. Rosenberger and G. L. Rorrer, Algal Res., 2013, 2, 16-27.
9. S. Boussiba, W. Bing, J.-P. Yuan, A. Zarka and F. Chen, Biotechnol. Lett., 1999, 21, 601-604.
10. F. Hempel, J. Lau, A. Klingl and U. G. Maier, PlosOne, 2011, 6, e28424.
11. K. Abe, K. Miyake, M. Nakamura, K. Kojima, S. Ferri, K. Ikebukuro and K. Sode, Microb. Biotechnol., 2014, 7, 177-183.
12. B. R. Cuenya, Thin Solid Films, 2010, 518, 3127-3150.
13. E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759-1782.
14. T. Klaus-Joerger, R. Joerger, E. Olsson and C.-G. Granqvist, Trend. Biotechnol., 2001, 19, 15-20.
15. K. B. Narayanan and N. Sakthivel, Adv. Colloid. Interfac., 2010, 156, 1-13.
16. S. Iravani, Green Chem., 2011, 13, 2638-2650.
17. S. A. Dahoumane, E. K. Wujcik and C. Jeffryes, Enzyme Microb. Tech., 2016, 95, 13-27.
18. S. A. Dahoumane, M. Mechouet, K. Wijesekera, C. D. M. Filipe, C. Sicard, D. A. Bazylinski and C. Jeffryes, Green Chem., DOI: 10.1039/c6gc02346k.
19. J. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, Small, 2007, 3, 672-682.
20. J. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, ACS Nano, 2007, 1, 429–439.
21. L. Castro, M. L. Blazquez, J. A. Munoz, F. Gonzalez and A. Ballester, IET Nanobiotechnol., 2013, 7, 109-116.
22. Y. Li, S. Tang, W. Song, Z. Zhu, X. Liu, X. Yan, C. Jin and Q. Ren, IET Nanobiotechnol., 2015, 9, 19-26.
23. J. Jena, N. Pradhan, B. P. Dash, P. K. Panda and B. K. Mishra, J. Saudi. Chem. Soc., 2015, 19, 661-666.
24. V. Patel, D. Berthold, P. Puranik and M. Gantar, Biotechnol. Report, 2015, 5, 112-119.
25. I. Barwal, P. Ranjan, S. Kateriya and S. C. Yadav, J. Nanobiotechnol., 2011, 9:56.
26. S. S. Sudha, K. Rajamanickam and J. Rengaramanujam, Ind. J. Exp. Biol., 2013, 52, 393-399.
27. K. S. Uma Suganya, K. Govindaraju, V. Ganesh Kumar, T. Stalin Dhas, V. Karthick, G. Singaravelu and M. Elanchezhiyan, Spectrochim. Acta A, 2015, 144, 266-272.
28. M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2006, 22, 2780-2787.
29. M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2007, 23, 2694-2699.
30. M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2006, 22, 7318-7323.
31. M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2007, 23, 8982-8987.
32. M. Mahdieh, A. Zolanvari, A. S. Azimee and M. Mahdieh, Sci. Ira. F., 2012, 19, 926-929.
33. L. Cepoi, L. Rudi, T. Chiriac, A. Valuta, I. Zinicovscaia, G. Duca, E. Kirkesali, M. Frontasyeva, O. Culicov, S. Pavlov and I. Bobrikov, Can. J. Microbiol., 2015, 61, 13-21.
34. D. MubarakAli, M. Sasikala, M. Gunasekaran and N. Thajuddin, Dig. J. Nanomater. Bios., 2011, 6, 385-390.
35. P. Roychoudhury, P. K. Gopal, S. Paul and R. Pal, J. Appl. Phycol., DOI: 10.1007/s10811-016-0852-1.
36. N. Chakraborty, A. Banerjee, S. Lahiri, A. Panda, A. N. Ghosh and R. Pal, J. Appl. Phycol., 2009, 21, 145-152.
37. D. Parial, H. K. Patra, A. K. R. Dasgupta and R. Pal, Eur. J. Phycol., 2012, 47, 22-29.
38. D. Parial, H. K. Patra, P. Roychoudhury, A. K. Dasgupta and R. Pal, J. Appl. Phycol., 2012, 24, 55-60.
39. J. Jena, N. Pradhan, R. R. Nayak, B. P. Dash, L. B. Sukla, P. K. Panda and B. K. Mishra, J. Microbiol. Biotechn., 2014, 24, 522-533.
40. D. Parial and R. Pal, J. Appl. Phycol., 2015, 27, 975-984.
41. S. Senapati, A. Syed, S. Moeez, A. Kumar and A. Ahmad, Mater. Lett., 2012, 79, 116-118.
42. C. Jeffryes, S. N. Agathos and G. Rorrer, Curr. Opin. Biotechnol., 2015, 33, 23-31.
43. R. Brayner, H. Barberousse, M. Hemadi, C. Djédjat, C. Yeprémian, T. Coradin, J. Livage, F. Fiévet and A. Couté, J. Nanosci. Nanotechno., 2007, 7, 2696-2708.
44. D. D. Merin, S. Prakash and B. V. Bhimba, Asian Pac. J. Trop. Med., 2010, 3, 797-799.
45. M. Mohseniazar, M. Barin, H. Zarredar, S. Alizadeh and D. Shanehbandi, BioImpacts, 2011, 1, 149-152.
46. S. A. Dahoumane, K. Wijesekera, C. D. Filipe and J. D. Brennan, J. Colloid Interface Sci., 2014, 416, 67-72.
47. S. A. Dahoumane, C. Djediat, C. Yéprémian, A. Couté, F. Fiévet, T. Coradin and R. Brayner, J. Nanopart. Res., 2012, 14:883.
48. L. M. Rösken, S. Körsten, C. B. Fischer, A. Schönleber, S. van Smaalen, S. Geimer and S. Wehner, J. Nanopart. Res., 2014, 16:2370.
49. S. A. Dahoumane, C. Yéprémian, C. Djédiat, A. Couté, F. Fiévet, T. Coradin and R. Brayner, J. Nanopart Res., 2016, 18:79.
50. T. Luangpipat, I. R. Beattie, Y. Chisti and R. G. Haverkamp, J. Nanopart. Res., 2011, 13, 6439-6445.
51. S. A. Dahoumane, C. Yéprémian, C. Djédiat, A. Couté, F. Fiévet, T. Coradin and R. Brayner, J. Nanopart. Res., 2014, 16:2607.
52. S. A. Dahoumane, C. Djédiat, C. Yeprémian, A. Couté, F. Fiévet, T. Coradin and R. Brayner, Biotechnol. Bioeng., 2012, 109, 284-288.
53. K. Govindaraju, S. K. Basha, V. G. Kumar and G. Singaravelu, J. Mater. Sci., 2008, 43, 5115-5122.
54. P. Roychoudhury, S. Ghosh and R. Pal, J. Plant Biochem. Biotechnol., 2016, 25, 73-78.
55. A. Schröfel, G. Kratošová, M. Bohunická, E. Dobročka and I. Vávra, J. Nanopart. Res., 2011, 13, 3207-3216.
56. C. Sicard, R. Brayner, J. Margueritat, M. Hémadi, A. Couté, C. Yéprémian, C. Djédiat, J. Aubard, F. Fiévet, J. Livage and T. Coradin, J. Mater. Chem., 2010, 20, 9342-9347.
57. C. Spedalieri, C. Sicard, M. Perullini, R. Brayner, T. Coradin, J. Livage, S. A. Bilmes and M. Jobbágy, J. Mater. Chem. B, 2015, 3, 3189-3194.
58. D. D. Lefebvre, D. Kelly and K. Budd, Appl. Environ. Microbiol., 2007, 73, 243-249.
59. D. MubarakAli, V. Gopinath, N. Rameshbabu and N. Thajuddin, Mater. Lett., 2012, 74, 8-11.
60. R. P. Mandal, S. Sekh, N. Sen Sarkar, D. Chattopadhyay and S. De, Mater. Res. Express, 2016, 3:055007.
61. S. A. Dahoumane, C. Djédiat, C. Yeprémian, A. Couté, F. Fiévet and R. Brayner, Thin Solid Films, 2010, 518, 5432-5436.
62. R. Brayner, C. Yeprémian, C. Djédiat, T. Coradin, F. Herbst, J. Livage, F. Fiévet and A. Couté, Langmuir, 2009, 25, 10062-10067.
63. R. Brayner, T. Coradin, P. Beaunier, J. M. Grenèche, C. Djédiat, C. Yeprémian, A. Couté and F. Fiévet, Colloid. Surface. B, 2012, 93, 20-23.
64. V. Subramaniyam, S. R. Subashchandrabose, P. Thavamani, M. Megharaj, Z. Chen and R. Naidu, J. Appl. Phycol., 2015, 27, 1861-1869.
65. V. Subramaniyam, S. R. Subashchandrabose, V. Ganeshkumar, P. Thavamani, Z. Chen, R. Naidu and M. Megharaj, Bioresour. Technol., 2016, 211, 698-703.
Recibido: 10 septiembre 2016
Aceptado: 20 noviembre 2016
Aceptado: 20 noviembre 2016
Si Amar Dahoumane,1* Mourad Mechouet,2 Francisco J. Alvarez,1 Spiros N. Agathos,1 Clayton Jeffryes.3*
1 School of Biological Sciences and Engineering, Yachay Tech University, San Miguel de Urcuquí, Ecuador
2 Laboratoire de Physique et Chimie des Matériaux, Université Mouloud Mammeri, Route de Hasnaoua,
BP 17 RP 15000, Tizi-Ouzou, Algeria.
3 Nanobiomaterials and Bioprocessing (NAB) Laboratory, Dan F. Smith Department of Chemical Engineering, Lamar University, Beaumont, TX, USA.
Corresponding authors:
SAD: sdahoumane@yachaytech.edu.ec
CJ: cjeffryes@lamar.edu
SAD: sdahoumane@yachaytech.edu.ec
CJ: cjeffryes@lamar.edu



















