Vol 7 No 3 2022- 40

Estimation of Epidermal growth factor (EGF), HER2, CA15-3 and Acid phosphatase in Iraqi breast cancer women
Ban Hussein Hameedi 1,4, Ali Abdul Al Hussain Mahdi 2. Ali Shalash Sultan3.
1 Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq.
2 Middle Technical University, College of Health and Medical Technology, Baghdad, Iraq.
3 Department of Science, College of Basic Education, Mustansiriyah University, Baghdad, Iraq.
4 University of Baghdad, College of Nursing, Department of Basic Science, Iraq, Baghdad.
Corresponding author: banhh@conursing.uobaghdad.edu.iq
Available from: http://dx.doi.org/10.21931/RB/2022.07.03.40
ABSTRACT
Breast cancer is one of frequent cancer that affects millions of people worldwide. Delayed diagnosis of these cancers has raised mortality and morbidity. Cancer biomarkers have tremendously increased the efficacy of treatment and the effectiveness of detection. This study aimed to investigate some biomarkers, including EGF, HER2, CA15-3, and Acid phosphatase, associated with early breast cancer (BC) diagnosis in Iraqi women. Carried on 90 Samples, the patients attended the Center for Early Detection of Breast Tumor at an oncology teaching hospital in Medical City. The study was conducted between 15/February (2021) and 20/July (2021). The consultant medical personnel made the diagnosis based on a Triple Assessment Technique, including physical breast examination, ultrasonography, with or without mammography and fine needle aspiration cytology. Female patients were divided into three groups (Benign, malignant and control). Benign B(34 patients) was split into subgroups, including. Benign premenopausal group B1(17 patients) Benign post-menopausal group B2(17patients) and malignant M(34 patients), malignant premenopausal group M1(17 patients) and malignant post-menopausal group M2(17 patients), and control group C include (11) premenopausal stage C1and (11) post-menopausal group C2. The value of EGF in Malignant cancer M1 (179.80 ±19.07) and M2(130.59 ±18.59)shows a highly significant (P≤0>05) increase in comparison with benign cancer and B2 and healthy control C1and C2 groups, respectively but B1 and B2 shows high significant (P≤0>05)decrease in comparison with C1 and C2 respectively. The values of HER2 show in B2(1.377±0.10); M1(11.76±0.10), and M2(11.79±0.09) increased significantly(P≤0.05) in comparison with C1, C2, B1 respectively. The values of CA-15-3 in M1 and M2 increase significantly(P≤0.05) compared with C1, C2, B1, and B2. The values of acid phosphatase in pre-and post-menopausal males in M1 and M2 increased significantly (p<0.05) compared with C1andC2.
Keywords: Epidermal growth factor, breast cancer, Acid phosphatase, HER2, CA15-3
INTRODUCTION
Breast cancer is the most frequent malignancy in women and one of this group’s leading causes of mortality. Breast cancer is a multifactorial disease¹, and different factors contribute to its occurrence. Although the disease occurs worldwide, its incidence, mortality, and survival rates vary considerably among the other parts of the world. More than half of the incidence of breast cancer and (60 percent) of deaths occur in low- and middle-income countries (LMICs)². Globally there is a vast difference in the age-standardized breast cancer incidence across the different populations. Women in Eastern Africa have a rate of 19.3 per 100,000, while women in Western Europe have a rate of 89.9 per 100,000².CA15-3 is a serological marker for monitoring the clinical course of BC patients. A persistent increase in the circulating concentration of this marker may suggest an inadequate response to cancer therapy in patients with metastatic BC. However, it has poor sensitivity, particularly at the early stages of the disease ³
CA15-3 can also be elevated in healthy individuals and in patients with benign conditions. It lacks the specificity needed for the cancer screening, diagnosis, staging, and/or sole use in monitoring post-therapy recurrence⁴. One of the most critical regulatory factors in the EGF family is the epidermal growth factor (EGF). EGF has been shown to successfully suppress follicular cell death and increase granulosa cell proliferation and oocyte maturation. And stimulate the ovulation process by binding to epidermal growth factor receptor (EGFR) ⁵. EGF is the prototypical member of a peptide growth factor family that induces the EGF receptors. The EGF/EGFR receptor signaling pathway plays an essential role in the differentiation, proliferation, and migration of different cell types, especially in epithelial cells. EGFR is expressed in 20 to 80% of breast carcinomas and leads to increased uncontrolled growth of cancer cells ⁶. HER2 is a growth-promoting protein on the exterior of all breast cells. HER2-positive breast cancer cells have more significant amounts of HER2 than normal⁷. HER2 overexpression stimulates the ligand-independent pathway HER2/HER3/PI3K complex formation and kinase activity in tumor cells, allowing trastuzumab resistance to be overcome by inhibiting PI3K, as well as gain of function mutations of PI3K and the loss of PTEN, Furthermore, overexpression of the insulin-like growth factor receptor 1 (IGF-1R) leads to sustained activation of the PI3K/Akt pathway, resulting in resistance to antihormonal and HER2-targeted therapies⁸
MATERIALS AND METHODS
Sample Collection
A short structured questionnaire was used to collect information on age, weight, marital status, height, number of pregnancies and children, average lactation term, age at first childbirth, family history of breast cancer or other cancers, first and second-degree relatives, age at marriage, age at menarche and menopause. (90) female patients coming to do an early examination for breast cancer and, after taking a history and doing a physical examination, verify that she is affected, whether it is a benign or malignant injury; we take a sample from the patients and conduct tests on it. Their ages ranged from 23-70 years. Female patients are divided into three groups (Benign, malignant and control). Benign B(34 patients) is divided into subgroups include Benign premenopausal group B1(17patients) and Benign post-menopausal groupB2 (17 patients) and Malignant M (34 patients), malignant premenopausal group M1(17 patients) and malignant post-menopausal group M2(17 patients),and control group includes (11) premenopausal group C1 and (11) post-menopausal group C2. The patients were referred to the Center for Early Detection of Breast Tumor at oncology teaching hospital in Medical city, during the period between 15/February (2021) and 20/July (2021). The consulting medical professionals established the diagnosis, which was based on a Triple Assessment Technique (i.e., physical breast examination, ultrasonography, with or without mammography and fine needle aspiration cytology) Demographical Samples were collected; venous blood. With respect to blood, 5 ml were collected in a 5ml disposable syringe, under aseptic conditions. The blood was put in a plain tube, and left for 15 minutes at 4ºC to clot. After that, it was centrifuged for 10 minutes at 5000 rpm.Sera were separated and kept in Apendrof tubes ,and then stored in deep freeze at(-20C˚) till examination for biochemical assay.
Measurement Epidermal growth factor (EGF)
Epidermal Growth Factor protein concentration was measured in the serum by using CUSABIO ELISA Kits. At the end of the assay, results are automatically calculated by an ELISA reader highly sensitive. Using a microplate reader set to 450 nm, the optical density of each well was calculated in just 5 minutes.
Normal values: premenopausal= 19.296- 230.904 pg/ml
Postmenopausal= 13.534-225.142 pg/ml
Measurement Human Epidermal Growth Factor Receptor 2(HRE2).
Human Epidermal Growth factor 2 Protein concentration was measured in the serum by using CUSABIO ELISA Kits. At the end of the assay, results are automatically calculated by an ELISA reader highly sensitive. Each well’s optical density was determined in five minutes by using a microplate reader set to (450 nm). If there is a wavelength adjustment option, select it (540 nm or 570 nm). Subtract the (540 nm or 570 nm) measurements from the readings at (450 nm). The plate’s optical flaws will be corrected by subtracting this amount. Readings taken on the spot (450 nm) without correction may be higher and less accurate.
Normal values: premenopausal= 0.596-1.023 ng/ml
postmenopausal =0.275-0.702 ng/ml
Human mammary carcinoma marker, cancer antigen(CA15-3)
Carcinoma marker 15-3 concentration was measured in the serum by using CUSABIO ELISA Kits. At the end of the assay, results are automatically calculated by an ELISA reader highly sensitive. Optical density was determined for each well within (10 minutes) by using a microplate reader set to (450 nm). If wavelength correction is available, set it to (630 nm). Subtract 630 nm observations from readings at other wavelengths (450 nm). This subtraction will eliminate any optical flaws in the plate. Without adjustment, readings taken at (450 nm) may be higher and less accurate.
Normal values: Premenopausal= 3.775- 16.999 u/ml
Postmenopausal= 2.633– 15.857 u/ml
Acid phosphatase (AP)
Acid phosphatase concentration was measured in the serum using Cayman chemical ELISA Kits. At the end of the assay, results are automatically calculated by an ELISA reader highly sensitive. Within 10 minutes, each well’s optical density was calculated by using a microplate reader set to (450nm).
Normal values: 41.395-68.882 pg/mL for premenopausal women.
29.417- 56.904 pg/mL for postmenopausal women.
Statistical Analysis:
The Statistical Analysis System-⁹ program was used to detect the effect of different factors on study parameters. Least significant difference –LSD test (Analysis of Variation-ANOVA) was used to compare between means significantly. Estimate the correlation coefficient between variables in this study.
RESULTS
The results were appeared in Fig (1).Shows the values of EGF show non-significant different (P≥0.05) in group C1(89.11 ±19.52) and C2(83.35 ±19.54 ),While the values in B1(27.54 ±4.51 );B2 (10.99 ±0.87 ), decrease significantly(P≤0.05) in comparison with C1,C2,M1 (179.80 ±19.07) and M2(130.59 ±18.59).M1 and M2 shows high significant(P≤0.05) in comparison with C1;C2;B1;B2 groups respectively. While Fig (2) summarized the values of HER2 in B1(1.066 ±0.04) show a non-significant (P≥0.05) difference in comparison with C1 (0.878 ±0.04 ), but it was increased significantly(P≤0.05) in comparison with C2(0.557 ±0.04).The values in B2(1.377±0.10);M1(11.76±0.10) and M2(11.79±0.09)were increased significantly(P≤0.05),in comparison with C1,C2,B1 respectively.
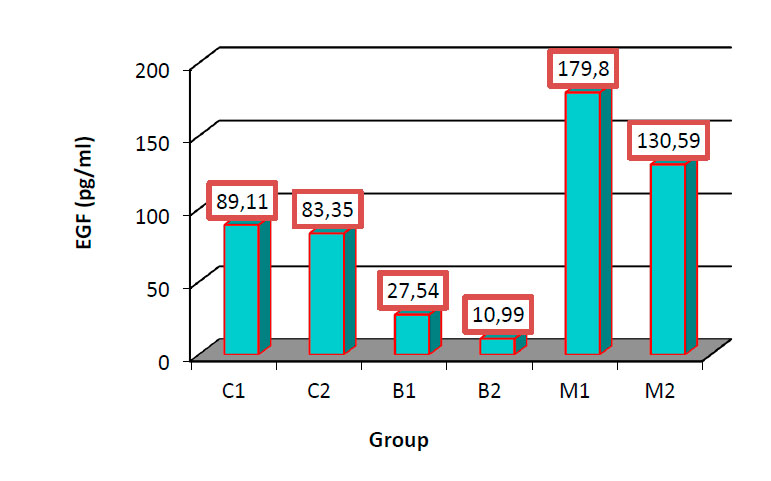
Figure 1. Comparison between different groups in EGF

Figure 2. Comparison between different groups in HER2
Fig (3) shows the values of CA-15-3 in C1(13.05 ±1.20); C2(11.91 ±1.20); B1 (7.61 ±0.65) and B2(12.92 ±1.25) show non -significant (P≥0.05) difference. M1(52.08 ±6.94)and M2 (44.42 ±5.60) increase significantly(P≤0.05) in comparison with C1(13.05 ±1.20),C2(11.91 ±1.20) ,B1(7.61 ±0.65 ),B2(12.92 ±1.25),But the values between M1 and M2 show non-significant difference(P≥0.05).

Figure 3. Comparison between different groups in CA-15-3
Fig(4).The values of acid phosphatase in B1(3.21 ±0.15) increased significantly(p<0.05)in comparison with C1(2.14 ±0.12),C2(1.133 ±0.12) while B2(2.78 ±0.23) was significant increase (p<0.05) with C2(1.133 ±0.12) . There was a non-significant (p>0.05) difference in values between groups B1 and B2. The values of acid phosphatase in M1(3.38 ±0.24) and M2 (2.93 ±0.27) increased significantly (p<0.05) in comparison with C1and C2.

Figure 4. Comparison between different groups in Acid phosphate
DISCUSSION
The group and M2 show high significance in the levels of EGF in comparison with other groups due to the malignant cell having irregular or mutation in the DNA to produce EGF protein; therefore, this parameter is used as a marker to diagnose breast cancer. EGF positively correlates with breast cancer cell proliferation ¹⁰ by binding with the EGFR. The fundamental ligand of these receptors is EGF, a potent mitogen. Especially in the cells overexpressing EGFR, which show in and M2. ¹¹well The endogenous manufacturing of epidermal growth factor (EGF) and insulin-like growth factor (IGF) polypeptide growth factors are strongly correlated to all the steps of breast cancer metastatic cascade and malignant transformation. Several studies have demonstrated that both estrogens and growth factors stimulate the proliferation of steroid-dependent cancer cells and that there are numerous levels of interaction between different signaling pathways. Importantly, estrogen receptor-positive breast cancer accounts for the majority of instances with a more favorable prognosis and pattern of recurrence, with endocrine therapy being the backbone of the treatment¹¹. This study showed an elevation level of HER2 in the M1and M2 group due to the association of this immune indicator with decreased immunity and the absence of estrogen, which causes cancerous cells in this category. This study agreed with¹²′¹³ studied the clinical significance of serum (sHER2) levels of patients with all stages of breast cancer. sHER2 levels were found to be raised in 15 percent (7/46) of stage I/II patients and 26 percent (9/35) of stage III patients. Stage I/II (P =0.002) and stage III (P =0.04) sHER2 levels predicted a poor prognosis. Two research found a significant correlation between high levels of sHER2 and worse disease-free survival(DFS) in patients with operable breast cancer(13). ¹⁴showed that high sHER2 levels were associated with poor prognosis in patients with early stage. Clinically, human epidermal growth factor receptor 2 (HER2) statuses of primary breast cancer tissue are used to determine biological subtypes, predict the outcome, and guide therapy decisions, particularly for the endocrine and HER2 targeted regimens ¹⁵. However, many studies have shown substantial discrepancies in ER, PR, and HER2 receptor profiles between primary and metastatic tumors. Based on a meta-analysis of about 39 studies, ¹⁶ reported. Conversion rates for ER, PR, and HER2 were 19.3 percent, 30.9 percent, and 10.3 percent, respectively. ¹⁷ showed similar findings in A meta-analysis of 47 studies involving 3,384 matched primary and metastatic breast cancer cases and found comparable results, with median ER, PR, and HER2-expression discordance rates of 14 percent, 21 percent, and10%, respectively.
The results suggest that the CA15-3 immunoassay in monitoring BC patients, especially for detecting early breast cancer disease in premenopausal and post-menopausal malignant. It is considered an immunological parameter that helps us to detect early cases of cancerous tumors because it is considered more sensitive and specific parameter than the rest of the immunological parameters.
In this study, serum CA15-3 had prognostic ability in primary early-stage and primary all-stage breast cancer patients. It’s unclear why CA15-3 can predict breast cancer outcomes. CA15-3 is the soluble form of the (MUC1), which may have something to link with MUC1’s function. ¹⁸. MUC1 has been shown to help cancer cells evade the immune system and accelerate cancer cell motility by activating certain membrane receptors¹⁸. Serum CA15-3 demonstrated a lower HR in metastatic studies than in primary investigations. Cancer antigen 15–3 (CA15-3, another name known as MUC1) is shaded by the tumor cells and is a well-known serological important marker for monitoring the clinical course of BC patients. A persistent increase in the circulating concentration of (CA15-3) marker may indicate an inadequate response to cancer therapy in patients with metastatic BC. However, it has poor sensitivity, particularly in the early stages of the disease¹⁹ CA15-3 can also be elevated in healthy people and patients with benign diseases, and it lacks the specificity required for cancer diagnosis, screening, staging, and post-therapy recurrence monitoring.¹⁹.The cause may be that CA15-3 is tightly corrected with tumor burden, and in pretreated tumors, it is consistently connected with tumor characteristics that can best predict the prognosis of survival²⁰. The tumor can be found at the primary stage in the developed countries (CA15-3), which is employed as a predictor, may be more beneficial in developing nations, and this phenomenon of region-specific tumor marker testing was also discovered by ²¹, though(CA15-3) seems to be able to assess the prognosis of the breast cancer, there was a lack of prospective study to clarify it, limiting its clinical utility. The levels of acid phosphatase increase significantly in premenopausal malignant M1 and post-menopausal malignant M2, and the premenopausal benign B1 and post-menopausal benign B2 increased considerably compared with C2. In general, this is not considered an accurate indicator of direct invasion with cancerous tumors but rather an indirect or accidental indicator because it is regarded as a symptom associated with this tumor during its inception.
The mechanisms for increased levels of these parameters in malignancy include humoral mechanisms fundamentally mediated by the parathyroid hormone-related peptide (PTH-rp), osteolytic bone in the concentration of the (ACP) particles which characterize metastasis and increased the lysosomes in the breast tumor cells²². In places where specific biochemical measures are not widely available, serum levels of these biochemical parameters could be used instead of traditional tumor indicators. ²³′²⁴. Increased level of (ACP) activity in breast tumors may be correlated to the amount of apoptosis that regulates tumor necrosis factor (TNF) that is going on in the cells ²⁴′²⁵′²⁶. ACP is a lysosomal enzyme found in most, if not all, lysosomes. Lysosomal enzymes are activated during the apoptosis in response to hormonal and other cellular triggers (acidic pH, lysosomotropic agents). These enzymes have been able to degrade all macromolecules and, in the end, destroy the entire cell²⁶′²⁷′²⁸.
CONCLUSION
We concluded from this present study that the women showing an increase in parameters EGF and HER2and CA-13 were high among malignant breast cancer compared with benign breast cancer, and acid phosphatase was increasing in pre and post-menopausal groups among harmless breast cancer women.
Acknowledgment: Thanks going for all who support us.
Conflict between authors: No conflict
Funds: self by authors
REFERENCES
1- Zendehdel M, Niakan B, Keshtkar A, Rafiei E, Salamat F. Subtypes of benign breast disease as a risk factor for breast cancer: A systematic review and meta-analysis protocol. Iranian journal of medical sciences. 2018 Jan;43(1);(1-8).
2- Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram‐Baptiste D, Saslow D, Wender RC. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: a cancer journal for clinicians. 2019 May;69(3):184-210.
3- Al-Azawi D, Kelly G, Myers E, McDermott EW, Hill AD, Duffy MJ, Higgins NO. CA 15-3 is predictive of response and disease recurrence following treatment in locally advanced breast cancer. BMC cancer. 2006 Dec;6(1):1-7.
4- Sturgeon CM, Hoffman BR, Chan DW, Ch’ng SL, Hammond E, Hayes DF, Liotta LA, Petricoin EF, Schmitt M, Semmes OJ, Soletormos G. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in clinical practice: quality requirements.
5- Cai Y, Lei X, Chen Z, Mo Z. The roles of cirRNA in the development of germ cells. Acta Histochemica. 2020 Apr 1;122(3):151506.
6- Schippinger W, Dandachi N, Regitnig P, Hofmann G, Balic M, Neumann R, Samonigg H, Bauernhofer T. The predictive value of EGFR and HER-2/neu in tumor tissue and serum for response to anthracycline-based neoadjuvant chemotherapy of breast cancer. American journal of clinical pathology. 2007 Oct 1;128(4):630-7.
7- Ismail R. SH., Issa AH and Almayah AA «Immunohistochemistry of Detection HER2 on Breast Cancer in Basra City». Sci. J. Med. Res. 2019;3(9):32-8.
8- Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers. 2018 Oct;10(10):342.
9- Cary N. Statistical analysis system, User’s guide. Statistical. Version 9. SAS. Inst. Inc. USA. 2012.
10- Skandalis SS, Afratis N, Smirlaki G, Nikitovic D, Theocharis AD, Tzanakakis GN, Karamanos NK. Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: focus on the role and impact of proteoglycans. Matrix Biology. 2014 Apr 1;35:182-93.
11-Voudouri K, Berdiaki A, Tzardi M, Tzanakakis GN, Nikitovic D. Insulin-like growth factor and epidermal growth factor signaling in breast cancer cell growth: focus on endocrine resistant disease. Analytical cellular pathology. 2015 Jul 15;2015.
12- Willsher PC, Beaver J, Pinder S, Bell JA, Ellis IO, Blamey RW, Robertson JF. Prognostic significance of serum c-erbB-2 protein in breast cancer patients. Breast cancer research and treatment. 1996 Oct;40(3):251-5.
13- Lee MH, Jung SY, Kang SH, Song EJ, Park IH, Kong SY, Kwon YM, Lee KS, Kang HS, Lee ES. The significance of serum HER2 levels at diagnosis on intrinsic subtype-specific outcome of operable breast cancer patients. PLoS One. 2016 Oct 5;11(10):e0163370.
14- Moreno‐Aspitia A, Hillman DW, Dyar SH, Tenner KS, Gralow J, Kaufman PA, Davidson NE, Lafky JM, Reinholz MM, Lingle WL, Kutteh LA. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2‐positive breast cancer receiving chemotherapy with or without trastuzumab: Results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer. 2013 Aug 1;119(15):2675-82.
15- Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, Senkus E, Cardoso F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). European journal of cancer. 2017 Apr 1;75:284-98.
16-Schrijver WA, Suijkerbuijk KP, Van Gils CH, Van Der Wall E, Moelans CB, Van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. JNCI: Journal of the National Cancer Institute. 2018 Jun 1;110(6):568-80.
17- Yeung C, Hilton J, Clemons M, Mazzarello S, Hutton B, Haggar F, Addison CL, Kuchuk I, Zhu X, Gelmon K, Arnaout A. Estrogen, progesterone, and HER2/neu receptor discordance between primary and metastatic breast tumours—a review. Cancer and Metastasis Reviews. 2016 Sep;35(3):427-37.
18-Li, H., Chen, K., Su, F., Song, E., and Gong, C. (2014). Preoperative CA 15-3 levels predict the prognosis of nonmetastatic luminal A breast cancer. The Journal of surgical research, 189(1), 48–56.
19- Terävä J, Tiainen L, Lamminmäki U, Kellokumpu-Lehtinen PL, Pettersson K, Gidwani K. Lectin nanoparticle assays for detecting breast cancer-associated glycovariants of cancer antigen 15-3 (CA15-3) in human plasma. PLoS One. 2019 Jul 25;14(7):e0219480.
20- Abdoli G, Bottai M, Sandelin K, Moradi T. Breast cancer diagnosis and mortality by tumor stage and migration background in a nationwide cohort study in Sweden. The Breast. 2017 Feb 1;31:57-65.
21- Ramsey SD, Henry NL, Gralow JR, Mirick DK, Barlow W, Etzioni R, Mummy D, Thariani R, Veenstra DL. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. Journal of Clinical Oncology. 2015 Jan 10;33(2):149.
22- Ohsako T, Yamamoto Y, Kawazoe T, Inoue K, Nagamoto N, Yoshida Y, Nakahara O, Sakamoto N, Iwase H. Two cases of stage IV breast cancer with severe hypercalcemia. Gan to Kagaku ryoho. Cancer & Chemotherapy. 2006 Feb 1;33(2):223-6.
23- Chan DS, Norat T. Obesity and breast cancer: not only a risk factor of the disease. Current treatment options in oncology. 2015 May;16(5):1-7.
24- Adams LM, Warburton MJ, Hayman AR. Human breast cancer cell lines and tissues express tartrate-resistant acid phosphatase (TRAP). Cell biology international. 2007 Feb 1;31(2):191-5.
25- Ahmed, S. A. Biochemical Studies on Serum Acid Phosphatases in Patients with Breast Mass in Erbil City. Middle East Journal of Internal Medicine, (2017). 1(2), 3-6.
26- Mahdi AA, Al-Salmani TS, Al-Qaisi MM. The novel role of healing from bacterial infections of lower limb open fractures by X-ray exposure. International Journal of Microbiology. 2020 Mar 19;2020..
27- Jameel YM, Mahdi AA, Tektook NK. New Designing and using Electromagnetic Field Device for drawing bacterial growth map. Research Journal of Pharmacy and Technology. 2019;12(9):4493-5.
28- Watson J, Tuggle A. Periodontal health and the lifecourse approach in bioarchaeology. Dental Anthropology Journal. 2019 Jul 19;32(2):12-21.
Received: 7 January 2022 / Accepted: 2 May 2022 / Published:15 August 2022
Citation: Hameedi B H, Al Hussain Mahdi A A . Shalash Sultan A. Estimation of Epidermal growth factor (EGF), HER2, CA15-3 and Acid phosphatase in Iraqi breast cancer women. Revis Bionatura 2022;7(3) 40. http://dx.doi.org/10.21931/RB/2022.07.03.40



















