Vol 5 No 4 2020 – 22
REVISION/REVIEW
Therapeutic Approach For COVID-19 – Clinical Challenges And Implementation
Faizan Ahmad1*, Abhichandan Das2, Shariq Suleman3, Upasana Pathak2 and Sabiha Naaz4
Available from: http://dx.doi.org/10.21931/RB/2020.05.04.22
ABSTRACT
SAR-CoV-2 originated from China with first case reporting from Wuhan, has been declared as pandemic by WHO on March 11 2020, which has affected millions of people around the globe with 213 countries and territories infected worldwide. It has caused the death of around 0.6 million individuals with no specific or promising vaccines or treatment available until now to prevent COVID 19, which has been approved; this has led the world to a global crisis not only on the health front but also affected the economic sectors. Researchers across the globe are working around the clock with their level best to discover promising therapeutic approaches for COVID 19, but till now for the treatment, only 3 therapeutics have been approved, including dexamethasone in U.K. and Japan, Avigan in Russia, Italy, and China and remdesivir in Japan and Australia; also convalescent plasma therapy is seen to be effective in critical cases of COVID-19, however, there are limitations with the use of this plasma therapy like the time point of treatment, optimal dose as the dose may vary with number of patients the particular therapeutic effects of convalescent plasma therapy will be further explored in randomized clinical trials. Several complete multinational studies are investigating alternative therapies. More than 100 countries entered a cooperation to evaluate applicants for high-profile COVID-19 diagnosis with this most massive WHO’s cooperation. According to WHO’s information, there are 28 vaccine candidates under clinical evaluation, with 6 of them entering phase 3. This review emphasizes the allopathic approaches along with the Chinese herbal medicine for the prevention of COVID-19. This paper also includes a brief discussion on the vaccine and nutritional supplements.
Keywords: SAR-CoV-2, COVID-19, therapeutics, drugs, vaccines
INTRODUCTION
The novel coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2) is the epigenetic modification of COVID-19, which was first identified at the end of 2019 in Wuhan, China1,2. The SARS-CoV and SARS-CoV-2 spike proteins are 77.5% identical to the primary A.A. (amino acid) sequence and bind to ACE2 protein (human angiotensin-converting-enzyme -2) as RBD (Receptor Binding Domain) bind firmly to ACE2 receptors present in human and bat 3. Researchers found that SARS-CoV-2 RBD has a higher binding affinity than SARS-CoV RBD to receptor ACE2 3.
Due to the rapid increase in this CoV disease, on January, 31 World Health Organization (WHO) declared COVID-19 a public health emergency of international concern 2. The virus has infected >3 million people around the globe and killed > 2 lakh people worldwide 2. On March 11 2020, COVID-19 was declared as a pandemic disease by WHO 4. SARS CoV2 is mainly transmitted in humans to humans through coughing and sneezing, leading to severe complications like septic shock, coagulation dysfunction, ARDS with multiple organ failure 4. Looking into the rapid increase in patient numbers daily, required for promising therapeutic intervention 1.
In this review, almost all therapeutic approach is available for the COVID-19 treatment ranging from drugs to Chinese herbal medicine, vaccines and convalescent plasma therapy has been discussed 1,5.
Drugs and treatments available
COVID-19 has been considered one of the biggest challenges in the modern era faced by medicine. Scientists and researchers are continually seeking remedies and medications to save infectious people’s lives and maybe even avoid infection. Though F.D.A has yet not approved any drug completely, but It provided authorization for emergency usage to others, with some of them being widely used, others giving promising evidence, and few of them being used as a tentative alternative.
Some of the drugs have been discussed below –
ANTIMALARIAL
Choloroquine and Hydroxychloroquine
Mechanism of action – Inhibition of RNA and DNA polymerase enzymes and inhibition of ACE2 cellular receptor. It was initially used to treat malaria and later approved for other diseases such as lupus, and rheumatoid arthritis, a less toxic version of chloroquine is known as hydroxychloroquine. This drug proved as one of the most controversial drugs during the initial phase Scientists have found that both medications will inhibit the coronavirus from replicating in cells during the small studies but later in March, WHO launched a randomized clinical trial to check the effectiveness of the drug against COVID-19 6,7.
RNA DEPENDENT
Remdesiver (GS-5734)
Mechanism of action – Drug evades proofreading by viral exoribonuclease enzyme8.
It was the first drug to get the FDA’s emergency authorization; this was initially tested against Ebola and Hepatitis C. Initial findings from the trials which began this season indicated that the medication might shorten the recovery period for people hospitalized with Covid-19 from 15 to 11 days 8,9
Favipiravir
Mechanism of action – Inhibits viral RNA synthesis 9.
Initially used for influenza, shows mixed evidence in its use against COVID-19 during trials. Limited research carried out in March suggested the medication could be effective in expunging the coronavirus from the airway, although the findings of broader, well-designed clinical trials are still awaited 8,9
Ribavirin
Mechanism of action – It is a nucleoside inhibitor that stops viral mRNA capping and viral RNA synthesis 7,8,9.
The antiviral properties of Ribavirin on the immune system was 1st observed with the treatment of hepatitis. It was also used for the treatment during SARS and MERS outbreak, which significantly showed the viral load reduction when given individually or in other antiviral drugs. A phase 2 randomized trial was conducted for COVID-19 when a combination of drug, including ribavirin (oral nucleoside analog), interferon beta-1b (injectable), and lopinavir-ritonavir (oral protease inhibitor) was found to be effective in suppressing COVID-19 although this study had several limitations 9,10,11
SUPPORTIVE THERAPY
Tocilizumab
Mechanism of action – Inhibited IL-6-mediated signaling10
Tocilizumab belongs to the IgG1 class, a recombinant humanized monoclonal antibody, Aimed at both the soluble and the Interleukin’s membranous forms receptor 6 (IL-6). It was initially used to treat severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, and life-threatening cytokine release syndrome induced by chimeric antigen receptor T cell therapy. In a study, the patients were treated with Either intravenous or subcutaneous tocilizumab, and the quality of care relative to routine practice. The correlation with tocilizumab usage became greater during reviews of the total mortality risk separately, although the study had many limitations as the comparison was not randomized 11,12
Sarilimuab
Mechanism of action – Binds to membrane-bound receptors & soluble IL-6 receptors (sIL-6 R & mIL-6 R) 3.
Sarilimuab precisely binds to the IL-6 receptor and demonstrates to block IL-6-mediated signaling. IL-6 is an immune system protein developed in high quantities in rheumatoid arthritis patients and correlated with disease development, joint damage, and other systemic problems. It is being tested for it’s potential to suppress COVID-19-associated overactive inflammatory immune response based on reports of significantly elevated rates of IL-6 in seriously ill coronavirus patients. But during the phase 3 trial of sarilimuab (kevzara) in the U.S., it was announced that the use of this drug requires mechanical ventilation and did not fulfill its primary and secondary endpoints when compared with placebo; depending on this result, the trial has been stopped11
Siltuximab
Mechanism of action – Binds to both membrane-bound receptors & soluble IL-6 receptors (sIL-6R & mIL-6R) 12
Siltuximab is an IL-6 focused mAb approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency ( EMA), among other regulatory bodies, for the treatment of patients with multicentered Castleman disease (MCD) who are found to be negative in human immunodeficiency virus ( HIV) and human herpesvirus-8 (HHV-8). As it is an inhibitor of IL-6, Prevents the escalation of IL-6 in the cytokine storm responsible for getting the elevated CRP concentrations during COVID-19. However, the drug has been approved for several clinical trials but has not been approved by the FDA for complications related to COVID-1913.
Leronliamb
Mechanism of action – Leronliamb mitigate cytokine storm and enhance immune response 8
Leronlimab is a humanized IgG4 mAb research that blocks CCR5, a cellular receptor significant in HIV infection, tumor metastases, and other diseases like NASH. Recently, a small clinical study found that leronlimab would minimize viral load from plasma and help rebuild the COVID-19 patient immune system. Within seven days of diagnosis, seriously ill patients reported rapid immune regeneration and extubation, according to IncellDx;. However, this was a small-scale study, although extensive randomized placebo-based studies are also going on to evaluate drug efficacy, where it has shown positive results8.
Anakinra
Mechanism of action – Inhibit binding of IL-1 beta & IL-1 alpha to the IL-1 type-1- receptor (IL-1R1) 12.
Anakinra is a non-glycosylated recombinant 17 kD Human receptor antagonist IL-1, with a limited half-life about 3–4 hours, with a strong health profile. It has been used for the treatment of rheumatoid arthritis. In a study, it was observed that this drug had been proved effective in the hyperinflammatory phase of COVID-19 where the level of CRP decreases with anakinra treated patient, and the respiratory functions have also seen improving; although, it also had several limitations like all other drugs and immunomodulators being considered 13
SOME DIFFERENT DRUGS USED FOR TREATMENT OF COVID-19
Corticosteroids
Both advantageous and injurious clinical effects have been documented in patients with other pulmonary infections, using corticosteroids (mostly prednisone or methylprednisolone), but Corticosteroids were examined with contradictory findings in critically ill patients with an acute respiratory distress syndrome (ARDS). Seven randomized, controlled trials, which included 851 patients, assessed corticosteroid use in ARDS patients. A meta-analysis of these test results showed that corticosteroid therapy lowered all-cause mortality risk compared to placebo. The COVID-19 Treatment Guidelines Panel (the Panel) suggested using dexamethasone based on a preliminary report from the RECOVERY trial, for the patients who are mechanically ventilated or either require supplement oxygen. However, the patients that do not require supplemental oxygen should not be given dexamethasone. In case it is not available other alternative glucocorticoids such as prednisone, methylprednisolone, or hydrocortisone are recommended. However, there is a risk of adverse side effects with these drug-drug interactions that should be closely monitored 14.
Metronidazole
Metronidazole is an antibiotic category of nitro-imidazole that has the potential to cure infectious diseases which have also been used to cure COVID-19; the treatment with this drug to COVID-19 patients led to an appropriate cytokine reduction stated by Gharebaghi et al., the main concern here is the effect of this drug on the significant cytokine reduction. In a study, IL-12 has been proved as a marker that can further help researchers identify patients infected with COVID-19 during early infection, MTZ inhibits IL-12 from binding to the IL-12 receptors by altering the surface and volume of IL-12. Few other drugs such as metronidazole phosphate, metronidazole benzoate, acyclovir, tetrahydrobiopterin, and 1- [1- (2- hydroxyethyl) -5-nitroimidazole-2-yl] – N-methylmethanimine oxide have also been approved by the U.S. Food and Drug Administration (FDA) during their virtual screening as they work in the same manner like MTZ. Other reasons can be modifications in the position of methyl and hydroxyl functional groups in MTZ have triggered IL-12 active sites’ inhibition. Therefore, this drug can be hope in COVID-19 treatment 15.
Bronchodilators
Bronchodilators are the medicine that relaxes the lungs’ muscle and widens the bronchi, making patient breathing easier. Mainly there are three types of bronchodilators which are Beta 2 agonists, Anticholinergics, and Theophylline16.
It is useful in treating patients of COVID-19 who has a history of chronic obstructive pulmonary disease (COPD) or underlying asthma 3.
Anticoagulant
Anticoagulants are medicines that prevent blood clots. The most common anticoagulant is warfarin, and some different anticoagulants are rivaroxaban, dabigatran, apixaban, and edoxaban. The Anti-coagulation is not recommended for the COVID -19 patient who has confirmed Venous thromboembolism (VTE) 3.

Table 1. Clinical trials of drugs for COVID-19 17
Convalescent plasma therapy
Convalescent plasma is adoptive immunotherapy that was practiced in the 1918 influenza epidemic to save from viruses and protect the lungs from severe damage 18. In the 1918 crisis of influenza, A (H1N1) virus, early implementation of convalescent blood products decreased the absolute risk of pneumonia-related mortality up to 21% 19. Neutralizing antibodies adhere to the spike-protein virus, which contributes to confirmation of the spike and could cause the peculiar result of better access into human cells through the IgFc- receptor 20. Convalescent Plasma therapy is the antibody-dependent enhancement of entry (ADE). A convalescent plasma therapy blood sample is collected from the person who recovers from COVID-19 infection, and then serum is isolated from the sample 5. The separated serum containing antibodies generated against the target antigen is given in the infected person to combat the virus’s infection. As antibodies bind to the antigen, the immune response is neutralized or activated [29]. Hence, identifying the monoclonal-antibody of humans that neutralizes SARS-COV-2 5. Until now, there is no effective treatment for SARS-COV-2 because of the absence of evidence 22. Convalescent plasma therapy is effective as antibodies from convalescent plasma suppress viremia 22. Immunoglobulin’s or convalescent plasma is seen to be effective in critically ill patients. The convalescent treated patient has to stay in the hospital for a shorter period with a lower mortality rate in patients who are not treated with convalescent plasma 24. The primary immune response is usually response development in patients during 10 to 14 days, followed by virus clearance 22. According to the Public Republic of China National Health Commission, convalescent therapy diagnosis will fellow principle 1. First, the disease course should not extend 3 weeks, as well as the patient should have positive viremia or viral nucleic acid certified by clinical experts. Second, their plasma infusion dose is determined for critically ill patients according to the patient’s weight 200-500ml (45 ml/kg) and depends upon the clinical situation24. According to the study, 5 patients with a reported COVID-19 & ARDS (Acute Respiratory Disease Syndrome) case obtained convalescent plasma with 2 consecutive transfusions of 200 ml – 250 ml of convalescent plasma with a cumulative dose of 400 ml25. After convalescent plasma infusion within a 3day body temperature normalized in 4 to 5 patients, and the score of sequential organ failure access (SOFA) decreased 25. Patients were negative with SARS COV2 neutralizing antibodies within 12 days of transfusion, and 14 patients recovered from ARDS after 12 days of transfusion, and within the 2 weeks of treatment, 3 patients were weaved from ventilation 3. After convalescent plasma transfusion, there is a reduction in pulmonary lesions during chest C.T. examination of the patient. There are no severe adverse effects reported so convalescent plasma therapy could be successful in patients infected with SARS- COV-2 25.
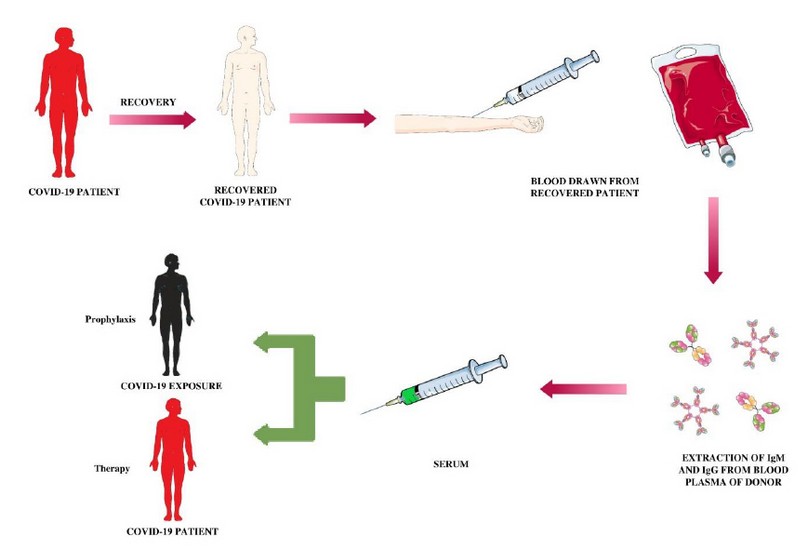
Figure 1. Convalescent plasma therapy path
Vaccine
Vaccines play a crucial role in not only protecting an individual but the entire community, as it trains the body’s immune system to identify and attack against the virus while encountering it. At present more than 100 projects are being worked on around the world for the COVID-19 vaccine. Many collaborations have formed around the globe, such as COVAX, with 150 countries involved. COVAX is co-led by Gavi, the Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO. Its goal is to promote the production and manufacture of COVID-19 vaccines and ensure equal and reasonable access for all countries globally. The ACT Accelerator is a ground-breaking global partnership to speed up the growth, output, and equal access to COVID-19 studies, treatments, and vaccinations 18,19,28,29
Several vaccines are under clinical and pre-clinical trials, and researchers are putting their best to discover a vaccine against this novel coronavirus. According to the document drafted by the world health organization, vaccines include 26 candidate vaccines under clinical trial with candidates including viral vectors, RNA, DNA, protein subunits; apart from these, 139 candidates are under pre-clinical evaluation. Some of the vaccines under clinical trial are discussed below.
RNA vaccine
There are 5 RNA vaccines under clinical trial currently with the first one being 1273 mRNA vaccine developed by Modern Tx Inc and NIAID (Cambridge, MA, USA) convert SAR COV2 viral protein sequence into mRNA when induced into the body 34,35. This vaccine is encapsulated within a lipid-nanoparticle and developed by the SARS- COV-2 virus against spike glycoprotein(s) where the administration route is intramuscular. The mRNA-1273 is under phase 3 clinical trial (NCT no.- NCT04470427). Another one is BioNTech/Fosun Pharma/Pfizer with vaccine candidate 3 LNP mRNAs being in phase 3 with the route of administration intramuscular; the rest are in phase 1 and 2 with trails still going on18,19,28,29.
DNA vaccine
There is 4 DNA vaccine with all of them being in phase 1/2 where vaccine candidates are DNA plasmid either with adjuvant or with electroporation with intradermal/ intramuscular route of administration18,19,28,29.
Virus-Like Particle Vaccine
There is only one VLP vaccine under clinical evaluation sponsored by Medicago Inc., where the candidate is plant-derived VLP adjuvanted with GSK or Dynavax adds administered through intramuscular route under phase 1 clinical trial18,19,28,29.
Protein Subunit Vaccine
There are 7 vaccine candidates out of which the one in phase 2 clinical trial is manufactured by Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd. Where vaccine candidate is Adjuvanted recombinant protein (RBD-Dimer) administered through the intramuscular route. The other 6 are either in phase 1/2 or in phase 118,19,28,29.
Non-Replicating Viral Vector Vaccine
There are 4 vaccines under clinical evaluation from which the one manufactured by the University of Oxford/AstraZeneca with vaccine candidate ChAdOx1-S is administered through intramuscular route in under phase 3 of the clinical trial while the rest is in phase ½. 18,19,28,29
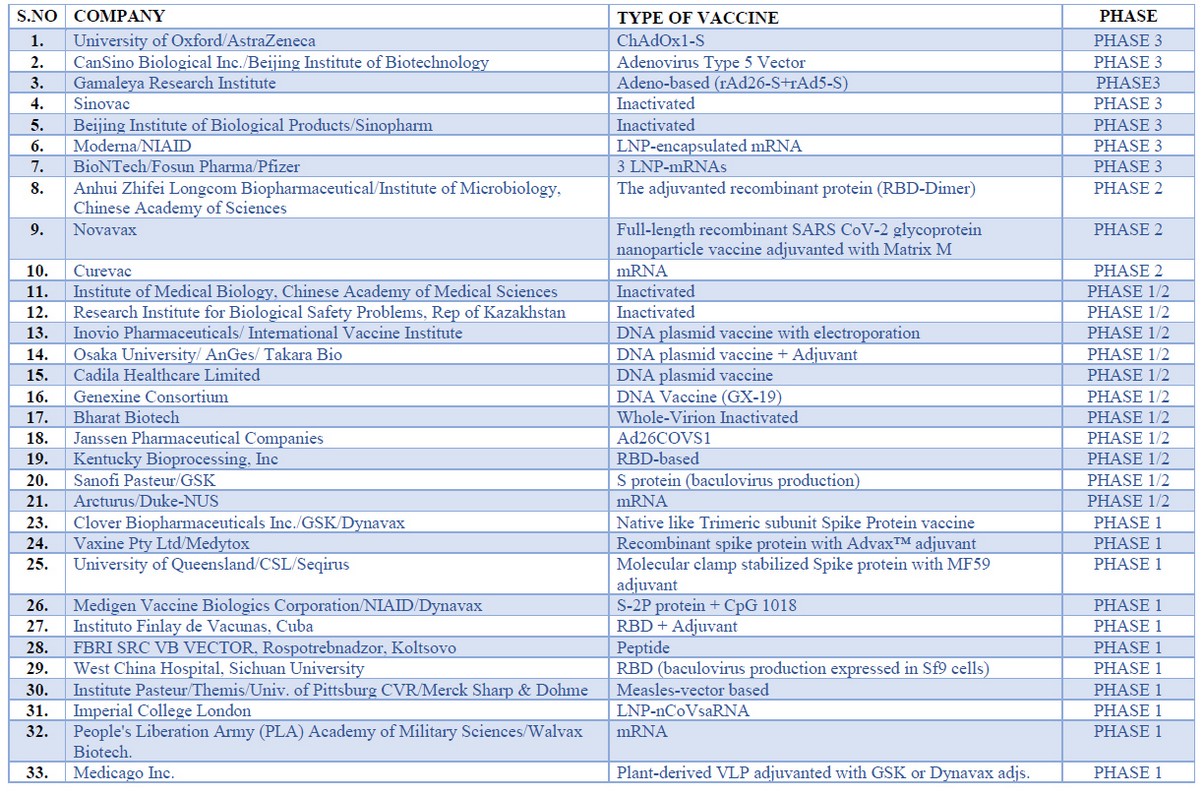
Table 2. Different types of vaccine for trial 28
Traditional Chinese Herbal (TCM) medicine
The government of China uses TCM (Traditional Chinese Medicine) with conventional medicine in approximately 26 provinces to treat COVID-19. On February 17, the NHC (National Health Commission) of the Public Republic of China treatment 85.20% of the total confirmed cases with TCM. Till March 1 2020, almost 50 trials (16.5%) out of 303 ongoing clinical trials include using TCM 14 cases (4.6%), including combined treatment with western medicine & TCM29,30. Below, the list of Traditional Chinese Medicine has been discussed.

Table 3. Different types of Chinese herbal medicine 29. 30
Micronutrients
Various micronutrients like iron, zinc, vitamin A, C, D, and selenium play a vital role in our immunocompetence 31,32. We will discuss some micronutrients that are essential for decreasing the risk of COVID-19 31,32.
Vitamin D
Vitamin D has the property to regenerate epithelial lining and can alleviate acquired immunity 27. Based on a meta-analysis, vitamin D plays a vital role in minimizing the alveolar damage during Acute Respiratory Tract Infection. Evidence showed that vitamin D has an almost 12% protective effect against viral and bacterial respiratory tract infection so, supplementation intake needs to be started before infection. The mechanism behind the use of Vitamin D lies in the stimulation of cathelicidins and defensins that we can increase the concentration of Anti-inflammatory cytokines and reduce the level of pro-inflammatory cytokines can induce inflammation and pneumonia. Vitamin D also decreases the replication of viruses, so, based on this data, vitamin D can play an influential role in decreasing the role of COVID-19 31,32.
Vitamin A
Vitamin A deficiency people are more prone to viral infection and various other influenza, measles, virus, etc. Vitamin A enhances the level and function of N.K. (Natural-Killer Cells), B-cells, T-cells, neutrophils, and macrophages or monocytes 31,32.
Vitamin C (L-ascorbic acid)
In early days literature, high doses of intravenous Vitamin C during sepsis during ARDS have a protective effect 31,32. HDIVC reduces DNA plasma cells, which play a significant role in sepsis-induced multiple organ failure inflammations. Thus, vitamin C can be useful micronutrients that can decrease the risk of COVID -19 31,32.
CONCLUSION
Global vaccination study and engineering initiative to tackle the pandemic COVID-19 had no history on pace and scale. The R & D area’s frequency suggests that vaccines under emergency usage or related protocols should be accessible by early 2021. It will mark a significant shift from the conventional vaccine delivery process, which requires more than 10 years on average compared to the rapid 5-year production cycle for the first Ebola vaccine. This novel vaccine production includes phases of concurrent and competitive growth, creative regulatory processes, and production capability scaling. Along with the vaccines, scientists and researchers worldwide are working day and night on drugs and immunotherapy that would be proven effective and efficient in fighting a pandemic number of drugs and immunomodulators under randomized clinical trials. However, none of them has got the approval from FDA, but these vaccines and drug can be the only effective way in which this world can combat this COVID-19 pandemic, which has affected not only the health sector but lead to a significant loss in the economic sector in countries around the globe.
Conflict of interest– The authors declare no conflict of interest
Funding – no source of funding
REFERENCES
1. Divya R, Santosh D. Therapeutic Application of Chloroquine and Hydroxychloroquine in Clinical Trials for COVID-19: A systematic review. medRxiv preprint doi:https://doi.org/10.1101/2020.03.22.20040964
2. Arun G, Margherita B, Dana S et al. Drug Development and Medicinal Chemistry Efforts Toward SARS-Coronavirus and Covid-19 Therapeutics Chem Med Chem doi : https://doi.org/10.1002
3. Tim Smith, PharmD, BCPS; Jennifer Bushek, PharmD; Aimée LeClaire, PharmD, COVID-19 Drug Therapy BCPS; Tony Prosser, PharmD Clinical Drug Information | Clinical Solutions
4. Shudong Z, Xialing G, Kyla G et al. Emerging Therapeutic Strategies for COVID-19 Patients Discoveries 2020, Jan-Mar, 8(1): e105 DOI: 10.15190/d.2020.2
5. Govindarajan K, Venkadapathi J, Saminathan R et al. A short review on antibody therapy for COVID-19New Microbes and New Infections S2052-2975(20)30034-2 https://doi.org/10.1016/j.nmni.2020.100682
6. Emanuele N, Nicola P , Tomasso et.al. National Institute for the Infectious Diseases «L. Spallanzani», IRCCS. Recommendations for COVID-19 clinical management Infectious Disease Reports 2020; 12:8543 doi:10.4081/idr.2020.8543
7. Binqing F, Xialing Xu, Haiming Wei Fu. J Transl Med (2020) 18:164 https://doi.org/10.1186/s12967-020-02339-3
8. Jahan S. Khalili , Hai Zhu , Nga Sze Amanda Mak et.al, Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID‐19J Med Virol. 2020;92:740–746.
9. Dimitar P Treatment of Covid-19 Infection. A Rationale for Current and Future Pharmacological Approach E.C. Pulmonology and Respiratory Medicine 9.4 (2020):38-58
10. Ritesh G ,Anoop M et.al, Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc) Diabetes & Metabolic Syndrome: Clinical Research and & Reviews 14(2020) 1871-4021
11. Giovanni Guaraldi*, Marianna Meschiari*, Alessandro Cozzi-Lepri et al., tocilizumab in patients with severe COVID-19: a retrospective cohort study Lancet Rheumatol 2020; 2: e474–84 Published Online June 24, 2020 https://doi.org/10.1016/ S2665-9913(20)30173-9
12. Reza G, Fatemeh H, Mohammad M et al. Metronidazole; a Potential Novel Addition to the COVID19 Treatment Regimen Archives of Academic Emergency Medicine. 2020;8(1): e40
13. Naidi Y, Han S Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19 International Journal of Biological Sciences 2020; 16(10): 1724-1731. doi: 10.7150/ijbs.45498
14. Corticosteroids Coronavirus Disease COVID 19 https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids
15. Zamora-Ledezma, C.; C., D.F.C.; Medina, E.; Sinche, F.; Vispo, N.S.; Dahoumane, S.A.; Alexis, F. Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives. Molecules 2020, 25, 4620.
16. https://www.nhs.uk/conditions/bronchodilators/9/4/2020Bronchodilators – NHS
17. Judith Stewart COVID-19: Prevention & Investigational Treatments
18. Miguel M. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus Antimicrobial Agents and Chemotherapy 64:e00399-20.https://doi.org/10 1128/AAC.00399-20.
19. Anne C , Hui G , David K . Treatment of COVID-19: old tricks for new challenges . Critical care (2020) 24:91 https://doi.org/10.1186/s13054-020-2818-6
20. COVID-19: Clinical information and treatment guidelines FIP Health Advisory 1-13
21. Cynthia L, Qiongqiong Z, Yingzhu Let al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Disease ACS Central Science .2020,6,315-331
22. Yuxin Y, Woo S, Yoong P The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment Int. J. Environ. Res. Public Health 2020, 17, 2323; doi:10.3390/ijerph17072323
23. Wen C, Ulrich S, Peter H et al. Current Tropical Medicine Reports https://doi.org/10.1007/s40475-020-00201-6
24. Yang Y, Md I , Jin W et al. Int. J. Biol. Sci. 2020; 16(10): 1708-1717. doi: 10.7150/ijbs.45538
25. Deng Z , Kun W, Xue Z et al. Journal of Integrative Medicine In silico screening of
Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus
26. Amin G, Sadaf N, Torsak T et al. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic Clinical Immunology https://doi.org/10.1016/j.clim.2020.108409
27. Erin M, Jason P Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options DOI: 10.1093/ofid/ofaa105
28. Zamora-Ledezma, C.; C., D.F.C.; Medina, E.; Sinche, F.; Santiago Vispo, N.; Dahoumane, S.A.; Alexis, F. Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives. Molecules 2020, 25, 4620
29. Jun R, Al Z, Xi W Traditional Chinese medicine for COVID-19 treatment Pharmacological Research 155 (2020) 104743
30. Chang L Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) Journal of Integrative Medicine https://doi.org/10.1016/j.joim.2020.02.004 2095-4964
31. Emanuela F. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 b. March 2020 Le Infezioni in Medicina, n. 2, 143-152, 2020
32. Pierre T, Xavier, Pascal M Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how doi: 10.1111/vox.12926
Received: August 21 2020
Accepted: October 21 2020
Faizan Ahmad1*, Abhichandan Das2, Sharique Suleman3, Upasana Pathak2, and Sabiha Naaz4
1* Department of Medical Elementology and Toxicology, Jamia Hamdard, Delhi, India
2 Centre for Biotechnology and Bioinformatics, Dibrugarh University, Assam, India
3 Department of Biotechnology, Jamia Hamdard, Delhi, India
4 Department of Biotechnology, Jamia Hamdard, Delhi, India
Corresponding author:Faizan Ahmad 1996faizanahmad@gmail.com



















