Vol 8 No 1 2023 – 69

Fadi S. H. AL-Sabagh1,* , Kais K. Ghaima2, Alhan H. Sh.AL-Dabbagh3.
1 Institute of Genetic Engineering and Biotechnology for postgraduate studies, University of Baghdad, Baghdad, Iraq.
2 Institute of Genetic Engineering and Biotechnology for postgraduate studies, University of Baghdad, Baghdad, Iraq.
3 Institute of Genetic Engineering and Biotechnology for postgraduate studies, University of Baghdad, Baghdad, Iraq.
* Correspondence: fadisalah_94@yahoo.com.
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.69
ABSTRACT
Multidrug-resistant Pseudomonas aeruginosa has emerged as a significant problem worldwide, posing a severe hazard to burn-infected patients. Antimicrobial peptides produced from humans or animals and synthetic peptides have received interest as antibiotic options for treating resistant bacteria, particularly those obtained from burn patients. The current work evaluated the role of antimicrobial peptide LL-37 as an antibacterial agent against multidrug P. aeruginosa isolates from burn infections. The study samples were collected between November 2021 and the end of February 2022 and included 157 clinical specimens as burn swabs from patients with burn infections admitted to four Baghdad hospitals in Baghdad, Iraq. The results of selective media, biochemical tests, and the ITEK2 system identified 39 isolates (24.8%) as p. aeruginosa from all collected bacterial cultures. The findings of the antimicrobial susceptibility test by disc diffusion method for the isolates under investigation revealed that P. aeruginosa clinical isolates were moderately resistant to antibiotics tested. Most P. aeruginosa isolates were highly resistant to Tetracycline (89.7%), Azithromycin (71.7%), and Amikacin, Cefepime, and Gentamycin. Also, the highest sensitivity was recorded for Ciprofloxacin, Piperacillin/tazobactam, C ceftazidime and Levofloxacin. The results of minimum inhibitory concentrations (MICs) of LL-37 against (8) multidrug-resistant P. aeruginosa isolates revealed that the concentration range of LL-37 was (15.6-1000 µg/ml), indicating that LL-37 has a significant effect on P. aeruginosa growth at low concentrations. In conclusion, t using the antimicrobial peptides LL-37 in treating life-threaded infections could lead to developing a new generation of antimicrobials that can overcome bacterial resistance mechanisms.
Keywords: Antibacterial, Burns, LL-37, Pseudomonas aeruginosa
INTRODUCTION
Bacterial infection is the most common complication and cause of death in burn patients because of the changes in the physiology of the skin. The patients are vulnerable to infection and burn wound sepsis1. This is due to a loss of the skin’s environmental barrier function, predisposing these patients to microbial colonization and invasion by dangerous bacteria such as P. aeruginosa and Staphylococcus aureus2. Patients with severe burn injuries are vulnerable to Healthcare-Associated Infections (HAIs). They are more likely to develop multidrug-resistant (MDR) bacterial infections as their hospitalization duration increases3. Wound infection is the leading risk factor in patients with burns, and burn wounds caused by multidrug-resistant Pseudomonas aeruginosa are among the most challenging wound care management in hospitals4. Antimicrobial peptides (AMPs) are molecules found in various life forms with a broad spectrum of inhibitory activity against pathogenic microorganisms and are a promising treatment option for multidrug-resistant pathogens5. Antimicrobial peptide LL-37 is the only member of the cathelicidins family found in the human body. LL-37 is expressed and secreted in the human body’s various epithelial cells, immune cells, body fluids, trauma secretions, etc. It can exert antimicrobial activity, participate in the immune regulation of the body, and promote wound repair. It is an active small molecule peptide with multiple functions6. Studies have shown that LL-37 has a broad antibacterial spectrum, such as antibacterial, antifungal, antiviral, antiprotozoal, and other antimicrobial effects7,8. Its mechanism of action is based on the bond between its positive charges and the negative charges of the phospholipids in the bacterial membrane. Bacterial lysis occurs due to altered permeability of the bacterial membrane with transmembrane pores9. Because of the importance and widespread of these bacteria and the challenge of their antibiotic resistance, this study aimed to evaluate the role of the antimicrobial peptide (LL-37) as a therapeutic agent against multidrug-resistant P. aeruginosa isolated from burn patients.
MATERIALS AND METHODS
Isolation and identification of P. aeruginosa
This study was conducted in Baghdad hospitals (Al-Yarmook and Al-Imam ALI ), Iraq, between November 2021 and February 2022. P. aeruginosa was isolated using Cetramide agar, Blood agar, and McConkey agar. According to the manufacturer’s recommendations, biochemical tests with the VITEK 2 system (bioMerieux, France) were used to identify these isolates. A total of 39 isolates of P. aeruginosa were collected from patients with burn infections from 157 burn swabs.
Antibiotic Susceptibility Test
The disc diffusion method was used to test antimicrobial susceptibility. In brief, overnight growth of P. aeruginosa was prepared on McConkey agar and then resuspended in normal saline. The suspension’s turbidity was adjusted to 0.5 McFarland, and this suspension was used to inoculate on Mueller-Hinton agar (Oxoid) plates. The antibiotic discs used in this study were as follows: Amikacin (AK), Gentamicin (GM), Imipenem (IPM), Meropenem (MEM), Ceftazidime (CAZ), Aztreonam (ATM), Ciprofloxacin (CIP), Tetracycline (T), Azithromycin (AZM), Levofloxacin (LEV), Cefepime (FEP), and Piperacillin-tazo After incubating the agar plates at 37 °C for 24 hours, the inhibition zone was measured and interpreted by the percentage of susceptible, intermediate, or resistant isolates as defined by CLSI breakpoint interpretative criteria10.
Minimum inhibitory concentrations (MIC) of LL-37
The broth microdilution method was used to determine the (MIC) of the LL_37 peptide sing the 96-well microtiter plate. The working solution of both was prepared at 4000 µg/ml in DW.100µl of the designed peptide was introduced into the first wells in row A. Rows B-H in columns had 100 µl of the broth alone, except row A had 100µl of the prepared peptide and broth. Twofold serial dilutions using a micropipette were done systematically down the columns (from row A-H). 100 µl was removed from the starting concentrations in row A and transferred to the next row with the 100µl broth, properly mixed, and the procedure was repeated up to the last row (H) where the last 100µl was discarded. This brings the final volume in all the test wells with the peptide to 100 µl except the column with 200 µl of the broth that served as sterility control. 100µl of bacterial inoculum was transferred into all the wells except the negative control. Microtiter plates were incubated at 37oC for 18-20 hrs. After incubation, 20 µl of resazurin dye was added to all the wells and set for 30 minutes to observe any color changes. The Minimum Inhibitory Concentrations were determined visually in broth micro dilutions as the lowest concentrations of the peptide and antibiotic. No color changed from blue to pink in the resazurin broth assay11.
RESULTS
Isolation and Identification of P. aeruginosa
One hundred and seventy-five burn swabs from patients with burns were cultured for the detection of P. aeruginosa. Each sample under study was streaked on sterile cetrimide agar and MacConkey agar plates. The colonies on MacConkey agar were mucoid and negative for lactose fermentation. All positive samples were tested twice to be sure of the result.The confirmation of the identification of all P. aeuginosa isolates was done by the results of VITEK 2 System.
Antibiotic Susceptibility of P. aeruginosa
The antibiotics resistance and sensitivity of P. aeruginosa isolates using disc diffusion method was evaluated for all 39 isolates with 12 antibiotic discs, The results of the sensitivity test are shown in figure 1.According to the findings of this study, the highest percentage of sensitivity was for ciprofloxacin (64.1%), Ceftazidime (64.1%), and also piperacillin-tazobactam (64.1%), while the lowest percentage of antibiotic sensitvity against P.aeruginosa, was for tetracycline (10.2 % ),Amikacin and Azithromycin (20.5%) .In the case of antibiotics resistance, the highest percentage were demonstrated for Tetracycline (89.7%), Azithromycin(71.7%),and Amikacin(69.2%). Also, the isolates had a low resistance for Piperacillin-tazobactam (17.9%),Ceftazidime(28.2%) and Azteronam (35.8%).
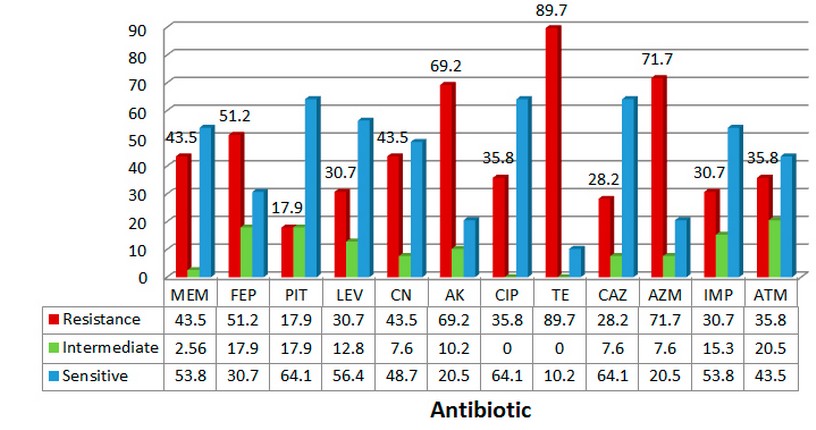
Figure 1. The percentage of antibiotic susceptibility of P. aeruginosa isolates from
Patients with burn infections.
The antibiogram results of multidrug-resistant P.aeruginosa showed that eight isolates were multidrug-resistant (29 isolates (74.3% ) were MDR, 6 isolates(15.3%) XDR and 4 isolates were sensitive to all antibiotics). The local study, which included a total of 524 burn and wound swabs collected from inpatients at Burn and Emergency Hospital, Duhok city, the Kurdistan region, Iraq, the results found that 60 (11.4%) isolates were identified as P. aeruginosa; 33 (55%) from female and 27 (45%) from the male. High resistance to piperacillin & ticarcillin (75% for both), ticarcillin/clavulanic acid (66.7 %) and tobramycin (63.6 %) were noticed in burn isolates. In the study on bacteriological profiles and antimicrobial resistance patterns of burn infections in the southwest of Iran, the cultures of various clinical samples obtained from 325 burn patients revealed that Pseudomonas aeruginosa was the most frequent bacterial isolate in Gram-negative bacteria and S. epidermidis was the most frequent species isolated in Gram-positive bacteria. We also found that 22% of strains were non-susceptible to meropenem and piperacillin-tazobactam. Ninety-nine (27%) of our collected isolates were resistant to multiple antibiotics.
Minimum Inhibitory Concentrations (MICs) of LL-37 against P.aeruginosa isolates
The results of the minimum inhibitory concentrations of the antimicrobial peptide LL-37 against eight multidrug-resistant isolates of P. aeruginosa using a microtiter plate assay with resazurin dye revealed that the range of inhibitory activity was (15.6-1000 µg/ml) and there was a difference in MICs between the isolates (Figure 2 and table 1). Some isolates, such as P2 and P13, were affected only by the concentration (15.6 µg /ml), while others such as P16, P19 and P30 were inhibited by (125 µg/ml). It was also discovered that the MICs of the isolates P1, P29, and P32 were 250, 62.5, and 62.5 µg /ml, respectively.
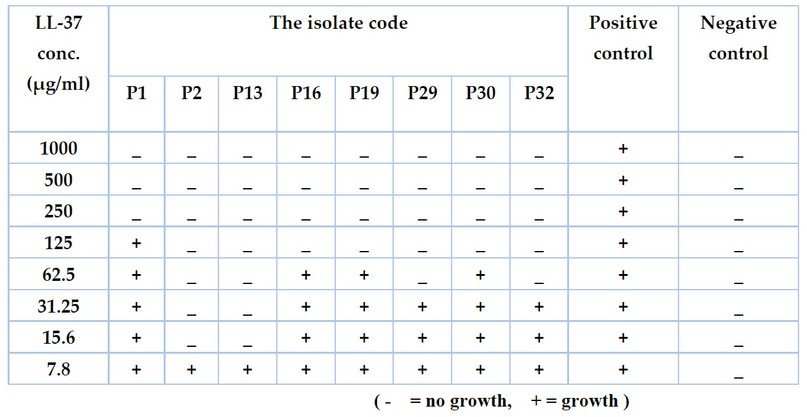
Table 1. The minimum inhibitory concentrations (MICs) of LL-37 against Pseudomonas auroginosa isolates at concentrations (7.8 -1000 µg/ml).
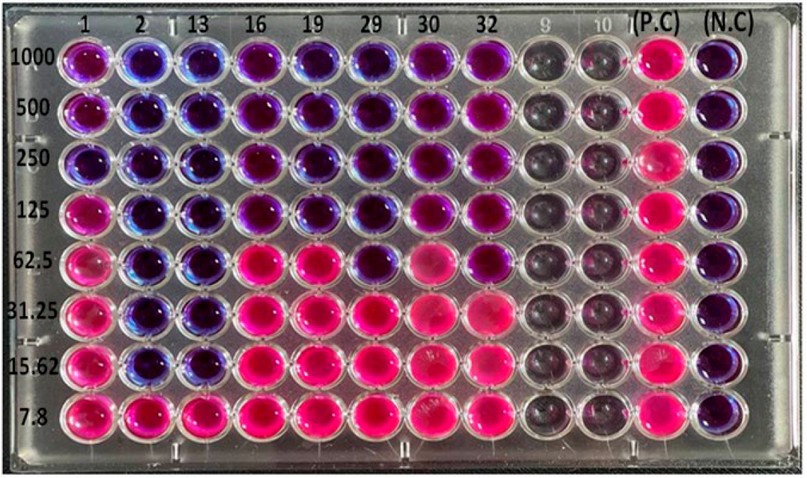
Figure 2. The minimum Inhibitory Concentrations (MICs) of LL-37 at concentrations (7.8 -1000 µg/ml) against Pseudomonas auroginosa Isolates by
Microtiter Plate Assay with Resazurin Dye.
The results18 of revealed that MIC of LL-37 against both P. aeruginosa reference strain (ATCC 15692 PAO1) and PA-ΔlasI/rhII was determined to be 256 µg/mL. LL-37 at sub-minimum inhibitory concentrations) had significantly downregulated the expression of 3 virulence factors (the expression of exotoxin A, elastase, and pyocyanin) and the expression of the Quorum sensing-related virulence genes of P. aeruginosa was significantly decreased, suggesting that LL-37 plays a vital role in the antibiofilm process. LL-37 can kill bacteria through direct antibacterial activities, as well as through immunomodulation. Like other AMPs, the primary mechanism of action is membrane disruption. The net positive charge of +6 allows for LL-37 to bind to the negatively charged membrane of bacteria. Upon binding, the introduction of transmembrane pores causes a disruption of cell integrity that leads to cell lysis and death.The single human cathelicidin peptide LL-37 has been shown to have antimicrobial and anti-biofilm activity against multiple Gram-positive and Gram-negative human pathogens and have wound-healing effects on the host.
DISCUSSION
The single greenish colonies were picked up in sterile cetrimide agar and slants for further characterization by different biochemical tests, such as oxidase, catalase, nitrate reduction, indole, and methyl red, Voges-Proskauer test, citrate utilization and glucose fermentation test 12,13. Colistin and piperacillin/tazobactam exhibited meager resistance 14. In a previous study on P.aeruginosa isolates from patients with burns infections, 50 P.aeruginosa strains were isolated, where the highest sensitivity of P. aeruginosa isolates was to colistin (100%) followed by polymyxin B (96%). In contrast, the lowest sensitivity was recorded against Ceftazidime (6%) 15. The maximum resistance was found to ampicillin, gentamicin, and ciprofloxacin, where the occurrence of multidrug resistance phenotype for P. aeruginosa was (30.3%) 16. The study of 17 P. aeruginosa isolates from the blood, and cerebrospinal fluid of patients from 10 countries during 2005–2017 demonstrated that the resistance to aztreonam (56%) was most common, followed by Levofloxacin (42%). The results of 18 revealed that MIC of LL-37 against both P. aeruginosa reference strains (ATCC 15692 PAO1). LL-37 has moderate antimicrobial activities against numerous Gram-negative and Gram-positive bacteria, including pathogens from the Pseudomonas, Escherichia, Staphylococcus, and Enterococcus genera 19. In addition, LL-37 can permeate the cell membrane to interact with intracellular targets, such as acyl carrier proteins 20. Combining the anti-biofilm effect and wound-healing properties of LL-37 may make it highly effective in resolving polymicrobial infected wounds when topically applied. It could be developed as a new therapeutic strategy to treat wound infections 21.
CONCLUSION
The antimicrobial peptide LL-37 has potent activity against antibiotic-resistant strains of P. aeruginosa even in low concentrations. This suggests that this peptide could be a significant step forward in developing new treatments for P. aeruginosa infection, particularly in burn and wound infections.
REFERENCES
1. D’Abbondanza, J.A.; Shahrokhi, S. Burn infection and burn sepsis. Surgical Infections,2021; 22(1), 58-64.
2. Shahrokhi, S. Initial Assessment, Resuscitation, Wound Evaluation, and Early Care. In Burn Care and Treatment, 2021; (pp. 1-12). Springer, Cham.
3. Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial infections after burn injuries: impact of multidrug resistance. Clinical Infectious Diseases,2017; 65(12), 2130-2136.
4. Jault, P.; Leclerc, T.; Jennes, S. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomized, controlled, double-blind phase, 2018; 1/2 trial. Lancet Infect Dis 3099:1–11.
5. Aguieiras, M.C.; Resende, LM; Souza, T.A.; Nagano, C.S.; Chaves, R.P.; Taveira, G.B.; Mello, É.O. Potent anti-candida fraction isolated from capsicum chinense fruits contains an antimicrobial peptide that is similar to plant defensin and is able to inhibit the activity of different α-amylase enzymes. Probiotics and Antimicrobial Proteins, 2021; 1-11.
6. Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013; 6:1543–75.
7. Raheem, N.; Straus, S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front Microbiol 2019;10:2866.
8. Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics (Basel). 2021; May 29; 10(6):650.
9. Handhal , N. A.; Ahmaed, A. S. . A Survey Study To Isolate Some Pathogenic Bacteria For Cooked Rice At Baghdad City. ). Journal of Life Science and Applied Research. 2020, 1, 54-59.
10. Clinical and laboratory standards institute, CLSI. performance standards for antimicrobial susceptibility testing. Second informational supplement. 2021; CLSI document M100-S22. Clinical and laboratory Standard.
11. Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Evaluation of the Antibacterial and Antifungal Properties of Phragmanthera capitata (Sprengel) Balle (Loranthaceae), a Mistletoe Growing on Rubber Tree, Using the Dilution Techniques. The Scientific World Journal, 2017;
12. Quinn, P.J.; Markey, B.K.; Leonard, F.C.; Fitz Patrick E.S.; Fanning, S.; Hartigan, P.J. Veterinary Microbiology and Microbial Diseases. 2nd ed. Ames, IA: Blackwell Publishing Ltd; 2011; pp. 287–290.
13. Carter, G.R.; Wise, DJ Essentials of Veterinary Bacteriology and Mycology. 6th ed. Iowa: The Iowa State Press; 2004; pp. 125–126.
14. Oumeri, M.M.Q.; Yassin, N.A. Molecular characterization of some carbapenem-resistance genes among Pseudomonas aeruginosa isolated from wound and burn infections in Duhok city, iraq. Journal of Duhok University,2021; 24(1), 136-144.
15. Al-Enzy, A. F. M., Saed, Z. J. M., Naser, A. S., Mohammed, Th. T., Abdulateef, S. M., Al- Khalani, F. M. H. & Abdulateef, F. M. The role of adding sodium chloride in broiler chicks diets to improve production performance and antioxidant status during heat stress. Annals of Tropical Medicine and Public Health. 2020, 23(16): 231- 612. Doi: 10.36295/ASRO.2020.231612
16. M. Ajeel, A.; A. Mehdi, L. . Effect Of Eruca Sativa Seeds Powder As Feed Supplementation On Some Physiological Traits Of Male Lambs. Journal of Life Science and Applied Research. 2020, 1, 20-30.
17. Nasrin, S.; Hegerle, N.; Sen, S.; Nkeze, J.; Sen, S.; Permala-Booth, J.; Choi, M.; Sinclair, J.; Tapia, M.D.; Johnson, J.K.Distribution of serotypes and antibiotic resistance of invasive Pseudomonas aeruginosa in a multi-country collection. BMC Microbiol. Jan 2022; 6;22(1):13.
18. Maysaloon W. Ibraheem, Abdulkhaliq A. Farhan, Sataa M. Salih, Th.T. Mohammed. Carcass characteristics of Awwasi lambs supplemented with Selenium and Vitamin D3. Iranian Journal of Ichthyology.2022, Vol 9, No. 1, pp: 355-359
19. Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Kamali Kakhki, R.; Safdari, H.; Khaledi, A.; Ghazvini, K. LL-37: Review of antimicrobial profile against sensitive and antibiotic-resistant human bacterial pathogens. Gene Rep. 2019;17:100519.
20. Khudair, M.Y Alyassein, R.N, Jasim, F.M. Improving the Quality of Ground Water in Some Areas of Al-Anbar Governorate by Recharging with Rainwater. IOP Conference Series: Earth and Environmental Science.2021, 761(1), 012009.
21. Duplantier, A.J.; van Hoek, M.L. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013; Jul 3;4:143.
Received: January 15, 2023 / Accepted: February 25, 2023 / Published:15 March 2023
Citation: AL-Sabagh F S H, Ghaima K K, Sh.AL-Dabbagh A H. The antibacterial activity of LL-37 peptide against multidrug-resistant Pseudomonas aeruginosa isolated from burn infections.
Revis Bionatura 2023;8 (1) 69. http://dx.doi.org/10.21931/RB/2023.08.01.69



















